Histo-Morphological Effect of The Small, Large Intestines and Stomach of Animal Models Treated With Aqueous Extract of Abelmoschus Esculentus
Abstract
This study investigates the effect of Aqueous extract of abelmoschus esculentus on the microanatomy of the small, large intestine and stomach and the body weight of Wister rats. Twenty-one adults male wistar rats weighing between 100-120 grams were assigned into three groups consisting of seven rats each; Group A (control), Group B (low dose), and Group C (high dose). The rats in the control group were fed with fed with feed and water only while the rats in groups B and C were treated with 0.1mg/kg body weight and 3.0mg/kg body weight of abelmoschus esculentus respectively for 14 days. At the end of administration, the final weights of all rats were recorded before sacrifice using cervical dislocation and the small, large intestine and the stomach were harvested, processed and stained using H&E stain. The results were revealed as significant (p<0.05) increased in the mean body weight compared with the weight in the control groups and experimental groups. The treated animal groups revealed increased cellularity, focal metaplasia of the mucosal cells with villous disruption in the small intestine and dysplasia of the mucosal with loss of epithelial shape in large intestine. The stomach histology showed gastric pits with goblet cells smooth muscles layer and surface epithelium in the control group. Sections from the low dose treated group showed deep epithelical gastric pit areas with marked depletion of pits and goblet cells while the high dose treated group revealed dysplasia of gastric pits, goblet cells and smooth muscles appear mildly eroded.
Author Contributions
Academic Editor: Raul Isea, Fundación Instituto de Estudios Avanzados -IDEA
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2022 Kebe E. Obeten, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Medicinal plants are the richest bio-resource of drugs of traditional systems of medicine, modern medicines, neutral ceuticals, food supplements, folla medicines, pharmaceutical intermediates, and chemical entities of synthetic drugs 1. Since prehistoric times, medical plants, also known as medicinal herbs, have been identified and employed in traditional medicine practices. Plants synthesis hundreds of chemicals compounds for functions including defence against insects, fungi, diseases and herbivorous animals, numerous phytochemicals with potential or established biological activity have been identified 2. Further, the phytochemical contents and pharmacological actions, if any, of many plants having medicinal potentials remain unassessed by rigorous scientific research to define efficacy and safety 3, 4.
The term “Medical plants” include various types of plants used in herbalism (“herbology” or herbal medicine”) 5. It is the use of plants for medicinal purpose, and the study of such plants like fruits, seed, stem, bark, flower, lead, stigma or a root as well as non woody plants, including those that came from tree and shrubs 6.
Medicinal plants are frequently employed in non-industrialized civilizations mostly because they are readily available and less expensive than modern medicine and in the year 2017, The total global export value of the thousands of varieties of plant with putative medical characteristics was estimated to be 2.2 billion dollars in 2012. the potential global market for botanical extract and medicine are estimated at several hundred billion dollars 7, 8, 9. Traditional medicine is still widely accepted as the preferred main health-care system in many communities, with over 60% of the world's population and around 80% of those in developing nations relying on medicinal plants directly for their medical needs. This is due to a variety of factors, including accessibility, affordability, and low cost 10, 11.
Okra (Abelmoschus esculentus), also known as gumbo or lady’s fingers, is a warm-season vegetable. It is a good source of minerals, vitamins, antioxidants, and fiber gumbo, is popular in the Southern United States, part of Africa and crop in many countries due to its nutritional value 12. Okra (Abelmoschus esculentus L.), belonging to the family malvacceae, is commonly known as lady’s finger, as well as by several vernacular names, including okra, blindi, okura, quimgombo, bamia, and lai long ma in the different geographical regions of its cultivation 13.
The okra plant (Abelmoschus esculentus) is a tropical and subtropical annual that is widely grown in Asia and Africa. Okra plants are native to Ethiopia, and by the 12th century B.C., they were being farmed by the Egyptians 14. Okra plants have sturdy stems, long bread serrated deeply lobed leaves and delicate yellow flowers marked with red or purple colour toward the base. Okra is comprised primarily of water, carbohydrates and proteins with very little fat and a fair amount of dietary fiber. Phytochemicals studies shows that okra pods contain flavonoids, tannins, steroids and triterpenes. Okra contains a moderate amount of oxalate a compound that is created by human body and is present in plants 15.
Okra is an excellent source of vitamin C and K1 Vitamin C is a water-soluble nutrient that contributes to our overall immune function while vitamin K1 is a fat-soluble vitamin that is known for its role in blood clotting. It is also rich in antioxidant that may reduce the risk of serious disease, prevent inflammation and contribute to heart and brain health. Okra contains a thick gel-like substance called mucilage, which can bind to cholesterol during digestion, causing it to be excreted with stools rather than absorbed into the body. Okra also contains a protein called lectin which inhibits the growth of human cancer cells 12.
The small intestine is about 20 feet (6 meters) long and folds many times to fit in the abdomen. Although it is longer than the large intestine, it is called the small intestine because it is smaller in width. The small intestine has three distinct regions – the duodenum, jejunum, and ileum. The duodenum, the shortest, is where preparation for absorption through small finger-like protrusions called villi begins. The jejunum is specialized for the absorption through its lining by enterocytes: small nutrient particles which have been previously digested by enzymes in the duodenum. The main function of the ileum is to absorb vitamin B12, bile salts, and whatever products of digestion were not absorbed by the jejunum. The length of the small intestine can vary greatly, from as short as 3.00 m (9.84 ft) to as long as 10.49 m (34.4 ft), also depending on the measuring technique used 16. The typical length in a living person is 3m–5m Tortora 17. The length depends both on how tall the person is and how the length is measured. Taller people generally have a longer small intestine and measurements are generally longer after death and when the bowel is empty.
The large intestine, also known as the large bowel, is the distal part of the gastro-intestinal tract and belongs to the digestive system in vertebrates. Water is absorbed here and the remaining waste materials are stored as faeces before being removed by defecation 18. The colon is the largest portion of the large intestine, so many mentions of the large intestine and colon overlap in meaning whenever precision is not the focus. Most sources define the large intestine as the combination of the cecum, colon, rectum, and anal canal 19. Overall, in humans, the large intestine is about 1.5 meters (5ft) long, which is about one-fifth of the whole length of the gastro-intestinal tract 20.
The stomach is the most dilated part of the digestive system, having a capacity of 1000–1500 ml in adult 21. It is located between the end of the oesophagus and the duodenums where the small intestine begins 22. It lies in the epigastric, umbilical, and left hypochondrial regions of the abdomen, and occupies a recess bounded by the upper abdominal viscera, the anterior abdominal wall and the diaphragm 21. However, borders are assigned by the attachment of the peritoneum via the greater and lesser omentum, thus dividing the stomach into an anterior and posterior surface 23. The principal function of the stomach is to mix the food with the gastric juice (acid, mucus and pepsin) and then release the resulting chyme, at a controlled rate into the duodenum for the process of absorption. Gastric motility is controlled by both neural and hormonal signals 21. Nervous control originates from the enteric nervous system as well as the parasympathetic (predominantly vagus nerve) and sympathetic systems 22. A number of hormones have been shown to influence gastric motility for example, both gastrin and cholecystokinin act to relax the proximal stomach and enhance contractions in the distal stomach 23. Other functions of the stomach include the secretion of intrinsic factor necessary for the absorption of vitamin B12 21.
Material and Method
Extract Preparation
The okra powder was dispensed in 15,000mls of distilled water in a plastic container. The mixture was rigorously stirred intermittently with a stick and allowed to stand for 24hours before it was filtered with a cloth sieve. The filtrates were evaporated at 50OC with water bath to obtain the crude solid extract and the extract obtained was stored in a refrigerator until the commencement of the administration.
Experimental Animal
Twenty-one (21) adults male wistar rats were purchased from the animal house of the Department of Human Anatomy, University of Cross River (UNICROSS) Okuku campus and were used for this study. The animals were distributed into three (3) groups of seven (7) animals for each group. The animals were housed in plastic cage under controlled light schedules (12-hours light & 12-hour dark cycle) and were fed with standard growers feed and water before the start of administration. They were weighed prior to the experiment.
Experimental Design
Twenty-one (21) adults male wistar rats were grouped in three groups of control, low and high dose according to their weight respectively.
Group A (control) animals received water and feed.
Group B (Low dose) animals received water and feed and aqueous extract of Abelmoschus esculentus at a dose of 1.0mls/kgBw.
Group C (High dose) animals received food, water and extract of Abelmoschus esculentus at a dose of 3.0mls/kgBw
Terminations of Experiment
At the end of the two (2) weeks period, animals in all groups were sacrificed a day after the end administration by cervical dislocation. The small, large intestine and stomach of animals from each group were removed and washed with 10% formalin.
Histological Studies
The small, large intestines and stomach of animals were removed and preserved in labelled bottles containing 10% buffered formalin. These were allowed to stand for 72 hours to achieve good tissue penetration and effective fixation. After these they were placed in ascending grades of ethanol for dehydration. First, they were treated with two changes of 70% ethanol each lasting for one (1) hour followed by 95% ethanol and then absolute alcohol for the same duration. Following dehydration, the tissues were cleared in three changes of xylene each lasting for thirty (30) minutes. Impregnation in molten paraffin wax at 58OC was carried out overnight and the following morning, the tissues were embedded in wax to form blocks. These blocks were trimmed and sectioned using a rotary microtome. The sectioning was floated in warm water (28OC) and taken up on albumised glass slides. They were air-dried and stain using the haematoxylin and Eosin 24.
Results
Morphological observation from the study shows an observable significant (p<0.05) increase in the final mean body weight when compared with the initial body weight observable in control vs low dose, control vs high dose and low dose vs control but not observable in low dose vs high does and high dose vs control dose. The final body weight of the control animals (133.9 ± 7.058) was significantly (p<0.05) higher than its initial body weight (111.4 ± 8.162). however, the mean final body weight of the low dose group (142.3 ± 4.716) and high dose group (145.7 ± 4.786) were significantly (p<0.05) higher than their initial body weights (128.0 ± 6.856) and (130.3 ± 6.157) respectively. Table 1, Figure 1
Figure 1.Effect of daily administration on body weight
| GROUPS | INITIAL | FINAL |
| Control | 111.4 ± 8.162 | 133.9 ± 7.058 |
| Low dose | 128.0 ±6.856 | 142.3 ± 4.71 |
| High dose | 130.3 ± 6.157 | 145.7±4.786 |
Histological Examination
Micrographs of The Small Intestine
Histological examination of the small intestine result in control animals revealed a normal architecture with the red pulp and white pulp with no pathological observation Figure 2. Administration of Abelmoschus esculentus at low dose shows an increased cellularity with numerous cells in the Mucosa and the villi projecting towards the lumen. Focal metaplasia of mucosal cells alongside villous disruption was also observed (Figure 3). The high dose group showed normal cellular architecture with no villous disruption (Figure 4).
Figure 2.Control showing the mucosa with intestinal villi (v) projecting towards the lumen (l) and the underlying smooth muscle layer (sm) all appearing normal
Figure 3.Low dose showing numerous epithelial cells in the mucosa (m) with villi projecting towards the lumen (l). The is focal metaplasia of the mucosal cells with villous disruption. smooth muscle layer appears normal. H & E. X40
Figure 4.High dose showing a normal mucosa (m) with villi (v) projecting towards the lumen(l) and underlying smooth muscle layer (sm). No pathology seen
Micrographs of The Large Intestine
Results from the large intestine histological observation showed normal appearance of epithelial cells lining the mucosa with no pathological observations in control animals (Figure 5). However, administration of Abelmoschus esculentus at low dose showed dysplasia of cells and loss of normal epithelial shape (Figure 6) and the high dose group showed mild dysplasia (Figure 7)
Figure 5.Control showing the lumen (l), the epithelial cells lining the mucosa (m) appearing normal H & E. X40.
Figure 6.Low dose showing the lumen (l), smooth muscle layer (sm) and dysplasia of the mucosa (m). there is loss of epithelial shape H & E. X40.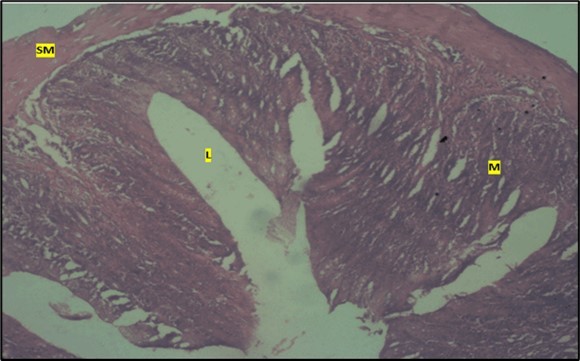
Figure 7.High dose showing the lumen (l), the smooth muscle layer(sm). mucosa (m) shows mild dysplasia H & E. X40.
Micrographs of The Stomach
Histological examination of the stomach in control animals showed normal appearance of epithelial cells lining the mucosa and normal smooth muscles in the submucosa (Figure 8). However, administration of Abelmoschus esculentus at low dose showed deep epithelial gastric area with marked depletion of pits and goblet cells (circled area) and normal smooth muscles in the submucosa (Figure 9) and the high dose group showed dysplasia of gastric pits with goblet cells in the mucosa and the smooth muscles in the submucosa were mildly eroded (Figure 10).
Figure 8.control plate showing gastric pits with goblets cells (g). Smooth muscle layer (SM) and surface epithelium (e) appears normal. (H&E).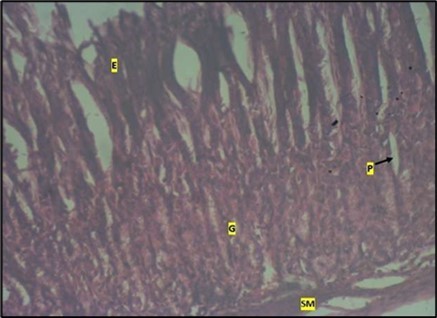
Figure 9.Image showing deep epithelial gastric pit area with marked depletion of pits and goblet cells (circled area). Smooth muscle (sm) appears normal in animals treated with Low dose of Abelmoschus esculentus stained with H & E technique X100
Figure 10.Image of a wistar rats stomach treated with high dose of abelmoschus esculentus showing dysplasia of gastric pits with the arrows mark (p). Goblet cells mark with arrow (g) appear at the basal mucosa and smooth muscle (sm) appear mildly eroded. The slide was stained using h & e technique. X100
Discussion
Since prehistoric times, therapeutic plants, often known as medicinal herbs, have been identified and employed in traditional medicine practices. Plants synthesis hundreds of chemicals compounds for functions including defence against insects, fungi, diseases and herbivorous animals, numerous phytochemicals with potential or established biological activity have been identified. Further the phytochemical contents and pharmacological actions, if any, of many plants having medicinal potentials remain unassessed by rigorous scientific research to define efficacy and safety 25. The World consumption of medicinal plants is growing rapidly. The need to verify claims of medicinal properties as well as determine their safety limits cannot be overemphasized. Usage of herbal preparations without control dosage coupled with non-availability of adequate scientific studies on their safety has raised concerns on their toxicity 26. Morphological findings from this present study, showed that there was a significant increase in the final body weights of rats treated with high and low doses of Albemoschus Esculentus extract when compared to rats in the control group. This is in conformity with previous studies by some authors reporting that the administration of abelmoschus esculentus causes increase in body weights of treated rats 27, 28, 29.
The significant increase in body weight may be due to the fact that, abelmoschus esculentus contains essential minerals, vitamins, lipids, amino acids including lysine and tryptophan and as well as proteins which are very vital for animal growth 30, 31, 32.
Histological Observations in the control group revealed a normal histomorphology of the cells in the mucosa with villi in the small intestine, lumen and epithelial cells lining the mucosa in large intestine and surface epithelium, gastric pits with goblet cells in the stomach. This study is similar to the findings of (Hoefling, et al. (2021) who looked at a deep learning-based model of normal histology of the small intestine 33.
In this present study, various histomorphological changes were observed in the small intestine, large intestine and stomach of treated rats. These were seen as increase cellularity (hyperplasia) in the mucosa and focal metaplasia of mucosal cells with villous disruption (in the small intestine), low grade dysplasia and loss of epithelial shape indicating cellular distortion (in the large intestine) and depletion and dysplasia of gastric pits and goblet cells respectively (in the stomach). Despite these, the smooth muscle layers in these three segments of the digestive tract investigated, showed normal histomorphology.
Abelmoschus esculentus contains various antioxidants including beta carotene, lycopene and vitamin E which have been used in the treatment of cancers 34. However, high doses of antioxidants including beta carotene have been implicated in increasing the risk of lung cancer and high dose supplements of antioxidants have been linked to health risks such as lung and prostate cancers 35, 36, 37.
Studies conducted in mice, shows that antioxidants cause cellular changes in such a way that, the spread of malignant melanoma becomes prominent 38. Antioxidants accelerate lung cancer progression 39.
Metaplasia is a tissue injury adaptation and it is a precursor to dysplasia-cancer sequence. Metaplasia is triggered by deleterious and inflammatory conditions 40. It is also as a result of trans-differentiation 41. Intestinal metaplasia is one of the risk factors of gastric epithelial dysplasia. Low grade dysplasia involves the growth of abnormal cells at a slow rate and has been observed to have a low risk of becoming cancerous because it usually changes back to normal through regression. .
The above reports and observations from previous studies relating to antioxidants and their implications in inducing histomorphological changes in animal systems, explains the underlying reason on the remarkable metaplasia and low-grade dysplasia which were seen in the small and large Intestines and stomach of rats treated with abelmoschus esculentus. This implies that, uncontrolled intake of abelmoschus esculentus may predispose consumers to various digestive tract precancerous conditions such as metaplasia and dysplasia, hence must be consumed with caution.
Conclusion
The results from this study shows that the prolonged intake of the extract at high dosage may cause adverse effect on the cells of the intestinal tract and stomach and could alter their functionalities while the moderate intake of the extract could promote a healthy intestine and stomach.
References
- 1.Alotaibi S, Alshoaibi D, Alamari H.et al.(2021). Potential significance of medicinal plants in forensic analysis: A review. , In Saudi Journal of Biological Sciences
- 2.Murugesan P. (2020) Phytochemical analysis and antimicrobial activity of edible lichen. , Journal of drug delivery and 10-22270.
- 3.Ekor M. (2014) The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Frontiers in pharmacology. 4, 177.
- 5.Mahomoodally M, Mooroteea K. (2021) A comparative ethno-religious study of traditionally used medicinal plants employed in the management of cardiovascular diseases. , Journal of Herbal Medicine 25, 100417.
- 6.Tripathi P, Shahin L, Sangra A.et al.(2019). Current status and future prospects for select underutilized medicinally valuable plants of Puerto Rico: A case study. , Cham, Medicinal 81-110.
- 7.Sofowora A, Ogunbodede E, Onayade A. (2013) The role and place of medicinal plants in the strategies for disease prevention. African journal of traditional, complementary and alternative medicines. 10(5), 210-229.
- 8.Awuchi C. (2019) Medicinal plants: the medical, food, and nutritional biochemistry and uses. , International Journal of Advanced Academic 5(11), 220-241.
- 9.Fokunang C, Ndikum V, Tabi O.et al.(2011). Traditional medicine: past, present and future research and development prospects and integration in the national health system of Cameroon. African journal of traditional, complementary and alternative medicines.
- 10.Mintah S, Asafo-Agyei T, Archer M.et al.(2019). Medicinal plants for treatment of prevalent diseases. Pharmacognosy-medicinal plants
- 11.Gemede H, Ratta N, Haki G.Woldegiorgis A.et al.(2015). Nutritional quality and health benefits of okra (Abelmoschus esculentus): A review. , J Food Process Technol 6(458), 2.
- 12.Elkhalifa A, Alshammari E, Adnan M.et al.(2021). Okra (Abelmoschus esculentus) as a potential dietary medicine with nutraceutical importance for sustainable health applications. 26(3), 696.
- 13.Chanchal K, Alok S, Kumar M.et al.(2018). A brief review on abelmoschus esculentus linn. okra. International journal of pharmaceutical sciences. 9(1), 58-66.
- 14.Islam M.Phytochemical information and pharmacological activities of Okra (Abelmoschus esculentus): A literature‐based review. , Phytotherapy research 33(1), 72-80.
- 15.John K DiBaise, Parrish C. (2016) Short bowel syndrome: practical approach to management. 31-9781498720809.
- 16.Kastl A, Terry N.Wu G et al .(2020). The structure and function of the human small intestinal microbiota: current understanding and future directions. Cellular and molecular gastroenterology and hepatology. 9(1), 33-45.
- 19.Ellis H.Mahadevan V.(2014). Anatomy of the caecum, appendix and colon. , Surgery (Oxford) 32(4), 155-158.
- 20.Krehbiel C, Matthews J. (2015) Absorption of amino acids and peptides (pdf). In D'Mello J. Amino acids in animal nutrition (2nd ed.) 41-70.
- 21.Lenglinger J. (2012) The cardia: esophageal or gastric? Critical reviewing the anatomy and histopathology of the esophagogastric junction". Acta chir iugosl. 59(3), 15-26.
- 22.Lahner E, Conti L, Cicone F.et al.(2019). "Thyro-entero-gastric autoimmunity: pathophysiology and implications for patient management. A review". , Best Pract Res Clin Endocrinol Metab 33(6), 101373.
- 23.Bronson K, Rotz S.D’Alessandro A.(2021). The human impact of data bias and the digital agricultural revolution. In Handbook on the Human Impact of Agriculture .
- 24.Mendes Lemos A, Machado N, Egea-Cortines M.et al.(2020). Assessment of quality parameters and phytochemical content of thirty ‘Tempranillo’ grape clones for varietal improvement in two distinct sub-regions of Douro. Scientia horticulturae. https://doi.org/10.1016/j.scienta.2019.109096
- 25.Abubakar A.Haque M.(2020). Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. pharmacy and bioallied sciences.https://doi.org/10.4103/jpbs.JPBS_175_19 , Journal of
- 26.Dubey P, Mishra S. (2017) A Review on diabetes and okra (abelmoschus esculentus). , Journal of medicinal plants 5(3), 23-26.
- 27.Majd N, Azizian H, Tabandeh M.et al.(2019). Effects of abelmoschus esculentus powder on ovarian histology, expression of apoptotic genes and oxidative stress in diabetic rats fed with high fat diet. Iranian journal of pharmaceutical research,18(1):. 369. PMCID: PMC6487426, PMID: 31089371.
- 28.Obeten K, Etukudo E, Anthony O.et al.(2022). Assessment of the vas deferens of abelmoschus esculentus (okra plant) treated wistar rats. , Journal of science, engineering and 9(1), 70-79.
- 29.Vermerris W.Nicholson R.(2006). Phenolic compounds and means of classification. Phenolic compound biochemistry.
- 30.Ciéslík E, Gebusia A, Fllipiak-Florkiewicz A.et al.(2012). The content of protein and amino acids in Jerusalem artichoke tubers (helianthus tuberous L.) of red variety rote zoenkugel. Acta Sci. Pol. Technol. Aliment,10: 433-441.
- 31.Alghamdi A.Nutritional assessment of different date fruits (phoenix dactylifera l.) varieties cultivated in Hail Province, Saudi Arabia. Bioscience biotechnology research communication,11: 263-269. , DOI: 10, 21786-11.
- 32.Hoefling H, Sing T.Hossain I.et al.(2021). A deep learning-based model of normal histology. , Toxicololgy 784-797.
- 33.Durazzo A, Lucarini M, Novellino E.et al.(2018). Abelmoschus esculentus (l): Bioactive components, beneficial properties- focused on antidiabetic role for sustainable health applications. Molecules,24: 38. PMID: 30583476. DOI: 10.3390/molecules 24010038.
- 34. (2013) National Centre for Complementary and Integrative Health. Antioxidants. In depth. NIH, Home-health information 1-6.
- 36.Middha P, Weinstein S, Mannisto S.et al.(2019). Beta carotene supplementation and lung cancer incidence in alpha-tocopherol, beta carotene cancer prevention study: The role of tar and nicotine. , Nicotine Tob 21(8), 1045-1050.
- 37.Melinda W. (2015) Antioxidants may make cancer worse. Cancer scientific African. New animal studies explain why supposedly healthy supplements like beta carotene could exacerbate a dread disease.www.scientificafrican.com/article/antioxidants-may-make-cancer-worse/.
- 38.Sayin V, Ibrahim M, Larsson E.et al.(2014). Antioxidants accelerate lung cancer progression in mice. , Sci. transl 6(221), 221-15.
- 39.Giroux V.Rustgi A.(2017). Metaplasia: tissue injury adaptation and a precursor to the dysplasia-cancer sequence. , Nature reviews 17(10), 594-604.
