Using A “Superrooting”Cultivar of Taxus Chinensis Var. Mairei to Unravel Antioxidative Enzymes’ and Micrornas’ Role on Adventitious Rooting
Abstract
Rooting of cuttings is very important for production of economically important plants. We produced thousands of plantlets in Taxus chinensisvar. mairei using the technology of rooting of cuttings and identified two types of rooted cuttings, one with low rate of root formation and another with high rate of root formation. To determine the physiological role of antioxidative enzymes and microRNAs during the process of rooting, we measured the levels of these antioxidative enzymes and microRNAs in the stem portion, needles, roots, and basal portion of cuttings. Compared to the cuttings with low rate of root formation, cuttings with high rate of root formation had higher expression of polyphenoloxidase (PPO), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APOX), glutathione reductase (GR), and superoxide dismutase (SOD) in the adventitious roots and basal portion of the rooted cuttings 77 days after planting. In the basal portion of cuttings, the content of thiobarbituric acid reactive substances (TBARS) and total phenols were decreased and the content of antioxidants was increased, but they did not changed in the needles of cuttings during planting. Analysis of microRNAs by quantitative realtime PCR demonstrated that expression of miR162, miR408, and miR857 increases in the basal portion of cuttings, but not in the stem portion of cuttings, 77 days after planting. Expression of miR408 and miR857 were also increased in the needles of cuttings 77 days after planting. Changes of these antioxidative enzymes and microRNAs associated with the rooting features of T. chinensisvar. maireicuttings and their functions have been discussed.
Author Contributions
Academic Editor: Mezni Ali, Department of Life Sciences, University of Carthag
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 Wei Tang, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Rooting of cuttings is very important for large-scale propagation of economically important plant species 1,2,3,4. Efficient propagation of these economically important plants is dependent on the efficiency of adventitious root formation in cuttings 5,6,7. Cuttings of Taxus chinensis var. mairei are recalcitrant to root. The detailed cause of their low rooting ability is not clear. In general, environmental conditions and endogenous biochemical compounds are considered to affect adventitious root formation 8,9,10,11. Among the endogenous compounds, proteins, enzymes, and phytohormones have been used for the efficient propagation of cuttings in many horticultural and forestry plants 9,12,13.
Taxus is a world wide economically important and an endangered gymnosperm genus 6,7,14,15,16,17,18,19. The barks of these plants are very valuable anticancer medicinal resource such as the powerful anticancer agent paclitaxel 20. Efficient propagation of these plants is critical to the scientific investigation and practical application in the field of conifer biotechnology 6,14,18,19,21. It has been reported that anticancer agents have been obtained in samples of Taxus wallichiana var. mairei 5 and Taxus x media var. Hicksii 6. In addition, the endophytic fungi in the bark of Taxus wallichiana var. mairei have been used to produce 10-deacetyl baccatin III 22. Investigations of transcriptome and metabolome in plants of Taxus genus demonstrated that difference in gene expression is related to the plant tissue type 23. However, biochemical and genetic studies in T. chinensis var. mairei are relatively rare because of lack of high efficient propagation system.
Antioxidative enzymes are known to affect morphogenesis in plants 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34. In the in vitro culture of many plant species, antioxidative enzymes can be biomarkers of adventitious root formation from microshoots 24,25 ,27,28,29,30,31. On the other hand, antioxidative enzymes have regulatory effect on adventitious root formation in shoot culture of a large number of plant species 35,36,37,38,39,40,41,42,43,44,45 and in Vitis vinifera cuttings 46,47,48,49. The above research results indicate that antioxidative enzymes are involved in adventitious root formation. Although the effect of some antioxidative enzymes adventitious root formation has been confirmed in plants 29,30,31,32,33, 50, 51 physiological role of polyphenoloxidase (PPO), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APOX), glutathione reductase (GR), and superoxide dismutase (SOD) during the process of in vitro rooting in T. chinensis var. mairei cuttings has not reported.
MicroRNAs are known to affect morphogenesis in plants 52,53,54,55. However, the effect of microRNAs on adventitious root formation might be more complicated because the composition and concentration vary with the species and growth phase 13,56,57,58,59,60,61,62. The functions of microRNAs and their mechanisms in regulating plant growth and development are still unclear 52, 53, 63, 64. Although the effect of some microRNAs on adventitious root formation has been confirmed and miR162, miR408, and miR857 have been reported to be involved in root initiation and growth in some plant species including model plants and crop plants 1,2,52,53,54,55,63,64,65,66 physiological role of microRNAs miR162, miR408, and miR857 during the process of in vitro rooting in T. chinensis var. mairei cuttings remains to be determined. Therefore, we examined the expression of miR162, miR408, and miR857 during the process of in vitro rooting in T. chinensis var. mairei cuttings.
The aim of this study is to elucidate the effect of antioxidative enzymes PPO, CAT, POD, APOX, GR, and SOD and microRNAs miR162, miR408, and miR857 on the rooting ability of cuttings of T. chinensis var. mairei. Expression levels of PPO, CAT, POD, APOX, GR, SOD, miR162, miR408, and miR857 in the stem portion, needle, roots, and basal portion of cuttings with high rate of root formation were compared with those of cuttings with low rate of root formation.
Materials and Methods
Plant Material and Cuttings
Taxus chinensis var. mairei genotypes Baokang was planted in a research field at Yangtze University and used in this experiment. Cuttings with 16 needles were obtained from these trees in early spring of the year and stored horizontally at 4oC in a plastic bag until cutting. Cuttings with one stem were prepared and cuttings with similar length and basal end diameter were planted in vermiculite in a planting box and placed in an unheated glasshouse. They were watered with 0.5-liter tap water per planting box every 3 days. Observations on rooting were made at 77 days after planting during each year. All cuttings with roots (>1 mm) were classified as rooted cuttings, and the roots (>1 mm) were used to prepare samples for analysis of antioxidative enzymes and micro RNA expression.
Sample Preparation for Antioxidative Enzymes and microRNAs Analyses
The number of cuttings used for antioxidative enzymes and microRNAs analyses were 756 (NR) and 649 (SR), respectively. The stem portion, needle, roots, and basal portions (2 cm) were collected from the cuttings on the planting day (day 0) and 77 days after planting. Each sample was chopped into small pieces with scissors, immediately frozen in liquid nitrogen, and then stored at −80oC until analysis. The levels of antioxidative enzymes and microRNAs were measured from samples of the stem portion, needles, roots, and basal portion of cuttings, respectively.
Thiobarbituric Acid Reactive Substances (TBARS) Determination
Lipid peroxidation was determined as the amount of thiobarbituric acid reactive substances (TBARS) measured by the thiobarbituric acid (TBA) reaction as described previously 67,68,69. Samples of stem portion, needle, roots, and basal portions (2 cm) of T. chinensis var. mairei cuttings were homogenized in 3 ml of 20 % (w/v) trichloroacetic acid (TCA). The homogenate was centrifuged at 2,655 g for 20 min and mixed with 20 % TCA containing 0.5 % (w/v) TBA and100 ml 4 % BHT in ethanol at 1:1. After the extracts of plant tissues were heated at 95 oC for 30 min, they were cooled on ice for 5 minutes, centrifuged at 10,000 g for 15 min. The absorbance the extracts of different samples was measured at 532 nm. The control of non-specific absorption at 600 nm was subtracted from the samples. The value of TBARS was calculated using the method described previously 26,67,70.
Measurement of Total Phenols
Analysis of phenols was conductedafter harvested the samples. Samples (1 g) of stem portion, needle, roots, and basal portions (2 cm) of T. chinensis var. mairei cuttings were homogenized in 50mL cold 80% methanol using a biomixer (Nihonseiki, Tokyo). Samples were washed twice with 50mL of 80% methanol and filtered. 150mL of distilled water was added to the samples to obtain a crude extract of the phenols.The total phenols content was determined using the Folin-Ciocalteu method 46,71,72,73]. After elimination of methanol in the crude extracts (180 mL) in vacuo, the aqueous phase was diluted five times with distilled water to the final volume of 900 mL. The diluted sample was mixed with 1N Folin-Ciocalteu reagent and then with 10% sodium carbonate 3 min later and placed at room temperature in darkness for 1 h. The absorbance of the reactants at 530 nm was measured. The total concentration of phenolic compounds was estimated by a standard curve obtained with chlorogenic acid (Wako Pure Chemical, Osaka).
Determination of Total Antioxidant Levels
Total antioxidant levels were determined from samples (300 mg) of stem portion, needle, roots, and basal portions of T. chinensis var. mairei cuttings using an antioxidant assay kit (Sigma–Aldrich) following the product manual. Antioxidant reactions were performed in a 96-well plate by reading endpoint absorbance at 405 nm using a plate reader (BioTek, Synergy 2, Winooski, VT, USA).
Measurement of Antioxidative Enzymes
Analysis of PPO, CAT, and POD activity was conducted by following the previous protocol 29,31,32,34,50,57,74,75. Samples (6 g) of stem portion, needle, roots, and basal portions of T. chinensis var. mairei cuttings were immersed in 100mL of cold 80% methanol containing 1mM butylated hydroxytoluene (BHT) and 0.01% ascorbic acid and stirred for 24 h at 4oC. Activity of antioxidant enzymes PPO was measured by following the modified methods previously described 34. The activity of CAT was determined spectrophotometrically as previously described 51. The decomposition of 1 mmol H2O2 per gram FW in 1 min was defined as one unit. The activity of POD was determined according to the procedure 32,51, with a Shimadzu UV-120IV spectrophotometer. The amount of POD catalyzing the oxidation of 1 mol guaiacol in 1 min was defined as one unit. The activities of APOX, GR, and SOD were determined as described previously 39,41,42,43. Two grams of control and sampes of stem portion, needle, roots, and basal portions of T. chinensis var. mairei cuttings were homogenized under ice-cold conditions in 3 ml of extraction buffer, consisting of 50 mM phosphate buffer (pH 7.4), 1 mM EDTA, 1 g PVP, and0.5 % (v/v) Triton X-100 at 4 oC. The extracts were centrifuged at 10,000 ´ g for 20 min. The supernatant was used to determine the enzyme activity. APOX activity was measured immediately in fresh extracts and was assayed as described 44. GR activity was determined by following the decrease in absorbance at 340 nm due to NADPH oxidation [42]. SOD activity was measured by the inhibition of the photochemical reduction of NBT, as described 39, 40.
Total RNA isolation and Quantitative RealTime PCR (qPCR)
Total RNA was isolated from frozen samples of stem portion, needle, roots, and basal portions of T. chinensis var. mairei cuttings using TRIzol reagent according to the manufacturer’s protocol (Invitrogen). The high quality cDNA were prepared using a TaqMan® MicroRNA Reverse Transcription Kit (Applied Biosystems). For miRNA expression analysis, TaqMan® MicroRNA Assays (Applied Biosystems) are carried out to amplify RNAs for quantitation of miRNAs. The controls used were as suggested by the manufacturer’s manual. The U6 gene was used as an internal control. Samples were examined in triplicate on the Applied Biosystems 7900HT System, according to the manufacturer’s manual.
Analysis of Expression of microRNAs
For the analysis of microRNAs (miR162, miR408, and miR857) in samples, the extraction procedure was used as previously described 26,27,53,54,55,58, 60,61,68,76. Two specific primers were used to amplify each of miRNAs. Primers used for Real-Time PCR are R1: 5’-AGTGGTTTATCGATCTCTTCCTTG-3’ and F1: 5’-GTGGTTCAAGCGTTTTATTGTTG-3’ for miR162, R2: 5’GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACCATGCT-3’ and F2:5’-TCAGCACAGGGAACAAGCAG-3’ for miR408, and R3:5’GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACATACAC-3’ and
F3: 5’-GCGGCGTTTTGTATGTTGAAG-3’ for miR857
Statistical Analyses
Mean values were used to determine the significant differences among different groups with the Least Significant Difference test at 5% level of probability. Statistic analysis of data was performed by Student’s t-test or one-way ANOVA using GraphPad Prism 6 (GraphPad Software Inc., CA).
Results
Adventitious Root Formation in Cuttings of Taxus chinensis var. mairei
During the in vitro rooting process in T. chinesis var. mairei, we used two different genotypes of T. chinensis, one with normal rooting and the other with super rooting phenotypes, which originated from sampling at two sites. Cuttings of T. chinensis with normal rooting (NR) are from BaoKang. Cuttings of T. chinensis with super rooting (SR) are from Jingzhou. 3436 cuttings of Baokang were planted at day zero and 756 cuttings of them actually rooted. 832 cuttings of Jingzhou were planted at day zero and 649 cuttings of them actually rooted at 77 days of in vitro rooting. Rooted cuttings (Baokang, 756 plantlets) has low rate of adventitious root formation [named normal rooting (NR) cuttings, Figure 1a], and another type of rooted cuttings (Jinzhou, 649 plantlets) has high rate of adventitious root formation [named super rooting (SR) cuttings, Figure 1b]. To determine the difference of these two types of rooted cuttings, percentage of rooting types (Figure 1c), number of adventitious roots per cuttings (Figure 1d), number of branch per cuttings (Figure 1e), and growth rate (Figure 1f) were measured. Our results demonstrated that percentage of rooting in super rooting cuttings is 3.9 fold higher than in normal rooting cuttings (Figure 1c). There are 3.7 fold increase in number of adventitious roots per cuttings (Figure 1d), 2.1 fold increase in branch number per cuttings (Figure 1e), and 2.6 fold increase in growth rate (Figure 1f) in rooted cuttings with high rate of adventitious root formation, compared to rooted cuttings with low rate of adventitious root formation. Our results indicated that number of adventitious roots (Figure 1d) and number of branch (Figure 1e), as well as growth rate (Figure 1f), are significantly increased in rooted cuttings with high rate of adventitious root formation 77 days after planting.
Figure 1.Adventitious root formation in cuttings of Taxus chinensis var. mairei. During the in vitro rooting process in T. chinesis, we identified two genotypes of rooted cuttings at 77 days of in vitro rooting. One type of rooted cuttings has low rate of root formation with low number of adventitious roots and lateral roots (Fig. 1a, normal rooting cuttings), another type of rooted cuttings has high rate of root formation with high number of adventitious roots and lateral roots (Fig. 1b, super rooting cuttings). Percentage of rooting types (Fig. 1c), Number of adventitious roots per cuttings (Fig. 1d), number of branch per cuttings (Fig. 1e), and growth rate (Fig. 1f) were measured for rooted cuttings. The statistically significant difference between groups was determined by one-way ANOVA. Data are presented as means of five independent experiments. Error bars represent standard error. The asterisk indicates significant differences compared to the rooted cuttings with low rate of root formation, as assessed by a t-test. ***P<0.001, significant relative to control. Vertical bars indicate standard error.
TBARS, Total Phenols, And Antioxidants Content in Shoot Cuttings
To evaluate the effect of TBARS, total phenols, and antioxidants content on root formation, TBARS, total phenols, and antioxidants content in different portions of rooted cuttings of T. chinensis var. mairei was determined at 0 and 77 days after planting.TBARS (Figure 2a, Figure 2b, and Figure 2c), total phenols (Figure 2d,Figure 2e, and Figure 2f), and antioxidants content (Figure 2g,Figure 2h, and Figure 2i) was measured from samples of stem portion, basal portion, and needles of rooted cuttings, respectively. Our results demonstrated that TBARS was significantly lower from day 0 to day 77 after planting in stem portion (Figure 2a) and basal portion (Figure 2b) of both NR cuttings and SR cuttings, total phenol was significantly higher from day 0 to day 77 after planting in stem portion (Figure 2d), but was significantly lower from day o to day 77 of planting in basal portion (Figure 2e) of both NR cuttings and SR cuttings. Content of antioxidants significantly increased in base portion (Figure 2h) of SR cuttings, compared to NR cuttings 77 days after planting. TBARS, total phenols, and antioxidants content were not changed in stem portion and needles of two types of cuttings 77 days after planting.
Figure 2.TBARS, total phenols, and antioxidants content of shoot cuttings of T. chinensis var. mairei at 0 and 77 days after planting. TBARS (Figs. 2a, 2b, and 2c), total phenols (Figs. 2d, 2e, and 2f), and antioxidants content (Figs. 2g, 2h, and 2i) was measured from samples of stem portion, basal portion, and needles of rooted cuttings. Experiment was repeated five times. The statistically significant difference between groups was determined by one-way ANOVA. Data are presented as means of five independent experiments. Error bars represent standard error. The asterisk indicates significant differences compared to the Phase I, as assessed by a t-test. *P<0.05, **P<0.01, ***P<0.001, significant relative to rooted cuttings with low rate of root formation. N.S., no statistics significance. Vertical bars indicate standard error.
PPO, CAT, and POD Activity in Cuttings
To examine the effect of PPO, CAT, and POD activity on root formation, PPO, CAT, and POD activity in shoot cuttings of T. chinensis var. mairei were measured at 0 and 77 days after planting. PPO (Figure 3a, Figure 3d, Figure 3g, and Figure 3j), CAT (Figure 3b,Figure 3e,Figure 3h, and Figure 3k), and POD activity (Figure 3c,Figure 3f, Figure 3i, and Figure 3l) were examined in stem portion, basal portion, needles, and adventitious roots of rooted cuttings of T. chinensis var. mairei at 0 and 77 days after planting. Our results demonstrated that activity of PPO, CAT, and POD was not changed in stem portion of cuttings between normal rooting and super rooting cuttings (Figure 3a, Figure 3b, Figure 3c). However, activity of PPO, CAT, and POD was significantly higher in basal portion (Figure 3d, Figure 3e, Figure 3f) and adventitious roots (Figure 3j, Figure 3k,Figure 3l) of SR cuttings, compared to NR cuttings 77 days after planting. Activity of PPO and CAT in needles of SR cuttings is similar to that of NR cuttings 77 days after planting (Figure 3g, Figure 3h). Activity of POD significantly increased in needles (Figure 3i) of SR cuttings, compared to NR cuttings 77 days after planting.
Figure 3.PPO, CAT, and POD activity in shoot cuttings of T. chinensis var. mairei at 0 and 77 days after planting. PPO (Figs. 3a, 3d, 3g, and 3j), CAT (Figs. 3b, 3e, 3h, and 3k), and POD activity (Figs. 3c, 3f, 3i, and 3l) in stem portion, basal portion, needles, and adventitious roots of rooted cuttings of T. chinensis var. mairei at 0 and 77 days after planting. Experiment was repeated five times. The statistically significant difference between groups was determined by one-way ANOVA. Data are presented as means of five independent experiments. Error bars represent standard error. The asterisk indicates significant differences compared to the rooted cuttings with low rate of root formation, as assessed by a t-test. *P<0.05, **P<0.01, ***P<0.001, significant relative to Phase I. N.S., no statistics significance. Vertical bars indicate standard error.
APOX, GR, and SOD Activity in Cuttings
To examine the effect of APOX, GR, and SOD activity on root formation, APOX, GR, and SOD activity were measured in shoot cuttings of T. chinensis var. mairei at 0 and 77 days after planting. APOX (Figure 4a,Figure 4d,Figure 4g, and Figure 4j), GR (Figure 4b, Figure 4e, Figure 4h, and Figure 4k), and SOD activity (Figure 4c, Figure 4f, Figure 4i, and Figure 4l) in stem portion, basal portion, needles, and adventitious roots of rooted cuttings of T. chinensis var. mairei at 0 and 77 days after planting. Our results demonstrated that activity of APOX, GR, and SOD in stem portion (Figure 4a, Figure 4b, Figure 4c) and needles (Figure 4g, Figure 4h, Figure 4i) of SR cuttings were similar to that of NR cuttings. However, activity of APOX, GR, and SOD in adventitious roots was significantly higher in SR cuttings (Figure 4j, Figure 4k, Figure 4l), compared to NR cuttings 77 days after planting. Activity of APOX, GR, and SOD in basal portion (Figure 4d, Figure 4e, Figure 4f) of NR cuttings was higher than SR cuttings before planting (0 day). Activity of APOX, GR, and SOD in basal portion (Figure 4d, Figure 4e, Figure 4f) of NR cuttings with was similar to that of SR cuttings 77 days after planting.
Figure 4.APOX, GR, and SOD activity in shoot cuttings of T. chinensis var. mairei at 0 and 77 days after planting. APOX (Figs. 4a, 4d, 4g, and 4j), GR (Figs. 4b, 4e, 4h, and 4k), and SOD activity (Figs. 4c, 4f, 4i, and 4l) in stem portion, basal portion, needles, and adventitious roots of rooted cuttings of T. chinensis var. mairei at 0 and 77 days after planting. Experiment was repeated five times. The statistically significant difference between groups was determined by one-way ANOVA. Data are presented as means of five independent experiments. Error bars represent standard error. The asterisk indicates significant differences compared to the rooted cuttings with low rate of root formation, as assessed by a t-test. *P<0.05, **P<0.01, ***P<0.001, significant relative to Phase I. N.S., no statistics significance.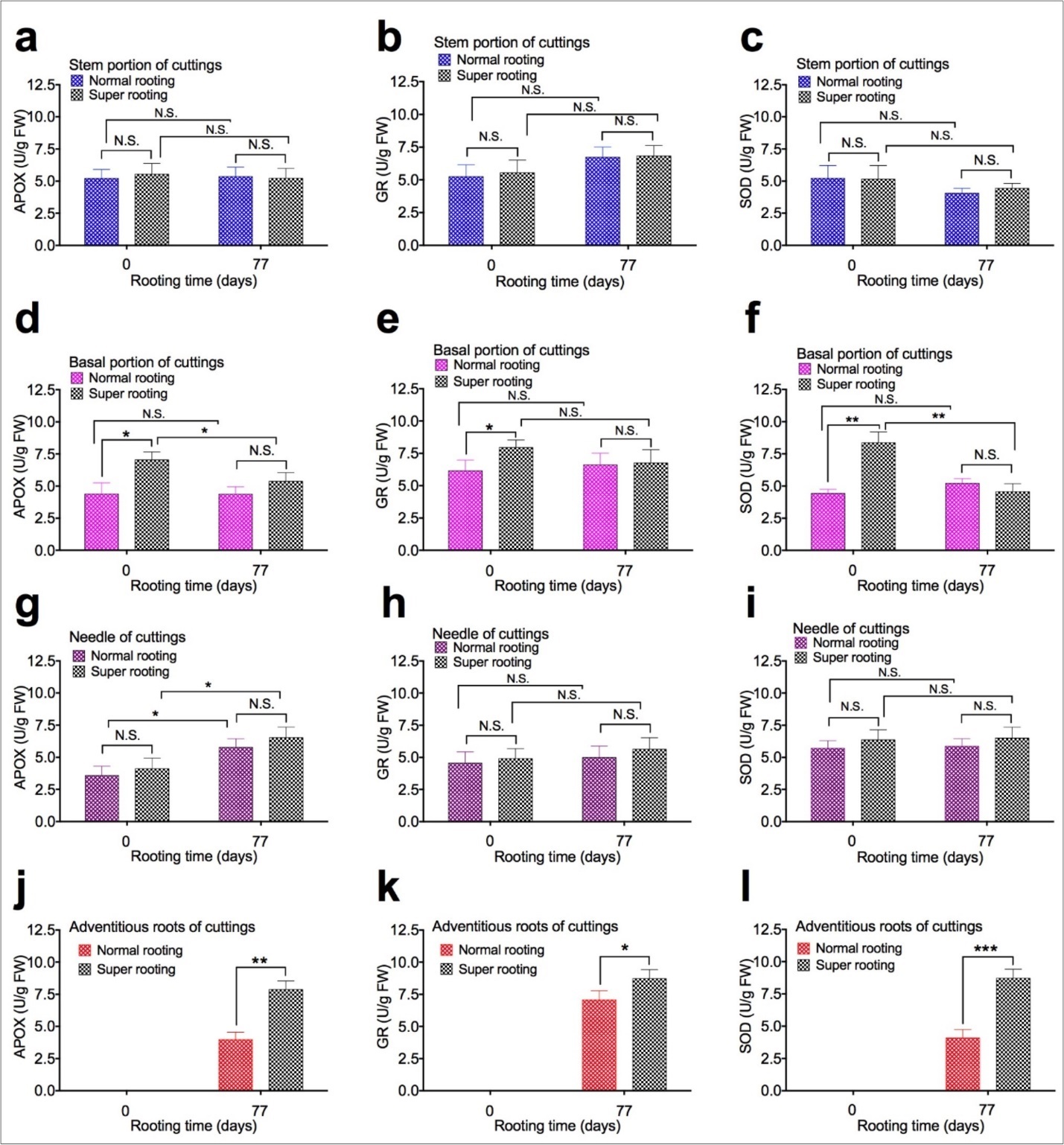
Expression of microRNAs in Cuttings
To examine the effect of expression of microRNAs on root formation, expression of microRNAs were measured in shoot cuttings of T. chinensis var. mairei at 0 and 77 days after planting. MicroRNA162 (Figure 5a, Figure 5d, Figure 5g, and Figure 5j), miR408 (Figure 5b, Figure 5e, Figure 5h, and Figure 5k), and miR857 (Figure 5c, Figure 5f, Figure 5i, and Figure 5l) in stem portion, basal portion, needles, and adventitious roots of rooted cuttings of T. chinensis var. mairei at 0 and 77 days after planting. Our results demonstrated that expression of miR162, miR408, and miR857 was not changed in stem portion (Figure 5a, Figure 5b,Figure 5c) of SR cuttings, compared to NR cuttings. However, expression of miR162, miR408, and miR857 significantly increased in basal portion (Figure 5d, Figure 5e, Figure 5f) of SR cuttings, compared to NR cuttings. Expression of miR162 was not changed in needles (Figure 5g) and roots (Figure 5j), but expression of miR857 significantly increased in needles (Figure 5i) and roots (Figure 5l) of SR cuttings, compared to NR cuttings 77 days after planting. Expression of miR408 was not changed in roots (Figure 5k), but significantly increased in needle (Figure 5h) of SR cuttings, compared to NR cuttings 77 days after planting.
Figure 5.Expression of microRNAs in shoot cuttings of T. chinensis var. mairei at 0 and 77 days after planting. MicroRNA162 (Figs. 5a, 5d, 5g, and 5j), miR408 (Figs. 5b, 5e, 5h, and 5k), and miR857 (Figs. 5c, 5f, 5i, and 5l) in stem portion, basal portion, needles, and adventitious roots of rooted cuttings of T. chinensis var. mairei at 0 and 77 days after planting. Experiment was repeated five times. The statistically significant difference between groups was determined by one-way ANOVA. Data are presented as means of five independent experiments. Error bars represent standard error. The asterisk indicates significant differences compared to the rooted cuttings with low rate of root formation, as assessed by a t-test. *P<0.05, **P<0.01, ***P<0.001, significant relative to Phase I. N.S., no statistics significance.
Discussion
Environmental conditions and endogenous biochemical compounds are important in regulating adventitious root formation. For example, the endogenous factor IAA regulates mitotic activity for root initiation in the cuttings of grape 49,72,73,77. The IAA level in grape cuttings at planting is higher in easy-to-root varieties than in difficult-to-root varieties 78,79,80. Increased IAA level of cuttings during planting is a more important factor for rooting than the higher IAA level at planting 8. High level of IAA was observed in the cuttings of chestnut with high rooting ability in the period of four days after planting 81, suggesting that accumulated IAA in basal portion of cuttings may regulate root architecture during rooting. Reduction of auxin signaling by the auxin antagonist α-(phenyl ethyl-2-one)-IAA rapidly decreases the expression of several core cell cycle genes 82,83. Overexpression of the mitotic cyclin CYCA2;3, which inhibits endocycle onset, promotes the termination of endoreplication 84. IAA signaling is critical for determining the timing of the transition to the endocycle 8,12,13,82. IAA plays a crucial role in stem cell specification in roots 83,84,85. In addition, reduction in RETINOBLASTOMA-RELATED protein (RBR) expression in Arabidopsis roots increases the number of stem cells without changing the duration of cell cycles in the meristem 86,87. Overexpression of RBR results in a loss of stem cells, indicating that an appropriate level of RBR is essential for maintaining the appropriate number of stem cells 86,87 indicating that the amount of IAA in basal portion of cuttings may affect rooting ability. Polyamines are another important endogenous factor in the maintenance of rooting ability in plants 26,69,70,76,88,89. It has been reported that an increase in free Put could be correlated with the formation of root primordial in cuttings of grape 73,78, and that Put may be a good marker for root differentiation 26,27. An increase in free Put levels was observed at an early stage of rooting of cuttings 26,27. Concentrations of Spd may affect root formation after planting in woody plants. It has been reported that the high level of Spd can be an indicator of the rooting ability of these species 25,26,27. Conjugated Put and Spd may have an effect on rooting. Conjugated Put and Spd were found to accumulate in Chrysanthemum leaf explants on a medium promoting root formation, followed by a decrease during the period of root emergence 25,26,27. In addition, changes in the levels were more rapid and substantial in the conjugated Put and Spd than in the free Put and Spd 25,26,27. Considering effects of auxin and polyamines on root formation have been extensively investigated in plants, we focus on influence of antioxidative enzymes and microRNAs on root formation in T. chinensis cuttings.
The rooting ability of T. chinensis cuttings depends on the varieties and cultivars. So far, little information on the rooting ability of T. chinensis cuttings is available. To analyze the effects of the endogenous factors on root formation in T. chinensis cuttings, we measured the levels of antioxidative enzymes and microRNAs in the stem portion, needles, roots, and basal portion of cuttings during the process of adventitious rooting in two cultivars of this species, known to have different adventitious rooting patterns. Our results demonstrated that antioxidative enzymes and microRNAs in the basal portion of the cuttings with high rate of root formation is an important factor affecting root formation in cuttings of T. chinensis. It has been reported in cuttings of some woody plants that formation of adventitious roots requires a certain level of antioxidative enzyme sand microRNAs 29,31,32,33,34,5, 1. The activity of different anti-oxidative enzymes like glutathione reductase (GR), superoxide dismutase (SOD), catalase (CAT), ascorbate peroxidase (APO), peroxidase (POD) and polyphenol oxidase (PPO) were examined in cultivars of tea 32 and wheat 29. These enzymes were reported to affect seed germination and root growth of several plant species including soybean roots 33. In the present study, we identified that activity of PPO, CAT, and POD was significantly increased in basal portion (Figure 3d, Figure 3e, Figure 3f) and adventitious roots (Figure 3j, Figure 3k, Figure 3l) of rooted SR cuttings, compared to NR cuttings 77 days after planting.
To counteract the effects of environmental stresses, the plants use antioxidant detoxification to avoid the accumulation of damaging free oxygen radicals and changes in the antioxidative machinery in plants under stress conditions played important role in root formation 39,40,41,42,43,44. POD, APX, GR, and lipid peroxidation are directly interrelated with different factors to modulate activities of various stress markers 39. The activities of antioxidative enzymes (SOD, CAT, POD, GR, APOX, MDHAR and DHAR) and contents of proteins and glutathione were increased in leaves of B. juncea plants after stress treatment 41. SOD, APOX, GR accumulated in plants at comparably higher levels than their counterparts under dry soil conditions in peanut 42. Inhibition of CAT, SOD, GPOX, APOX, GR, as well as increasing MDA concentration results in decreasing of fine roots volume 43. In the present study, we identified that activity of APOX, GR, and SOD in adventitious roots significantly increased in SR cuttings (Figure 4j, Figure 4k, Figure 4l), compared to NR cuttings 77 days after planting.
MicroRNAs are known to affect root formation in plants. In Chlamydomonas reinhardtii, expression of multiple microRNAs, miR397, miR408, and miR857, involved in copper homeostasis 53,54,55. In I. campanulata, miR398, miR168, miR858, miR162 and miR408 were up-regulated, while miR394 and miR171 were down-regulated under stress. In J. pentantha, miR394, miR156, miR160, miR164, miR167, miR172, miR319, miR395, miR396, miR403 were up-regulated and miR157 was down-regulated under stress. Basal miRNA levels and their drought-mediated regulation were very different between the two species 58,60,61. Plants utilize complex gene regulation mechanisms to tolerate abiotic stresses. MicroRNAs are important regulators of gene expression acting at post-transcriptional level. Expression levels of miR408 regulated drought responsive genes and rooting-related genes [90-94]. Expression of microRNAs is related to the nutrition. Upon N starvation, the expression of miR169, miR171, miR395, miR397, miR398, miR399, miR408, miR827, and miR857 was repressed, whereas those of miR160, miR780, miR826, miR842, and miR846 were induced. Among these N-starvation-responsive miRNAs, several were involved in cross-talk among responses to different nutrient (N, P, S, Cu) deficiencies. miR160, miR167, and miR171 could be responsible for the development of Arabidopsis root systems under N-starvation conditions 53,54,55,63,64. In the present study, we identified that expression of miR162, miR408, and miR857 significantly increased in basal portion (Figure 5d, Figure 5e, Figure 5f) of SR cuttings, compared to NR cuttings.
Conclusion
In the present study, changes in the levels of antioxidative enzymes and microRNAs during the process of rooting were observed in the cuttings of T. chinensis. Significant increase in antioxidative enzymes and microRNAs expression levels was found in basal portion, stem portion, tips of adventitious roots, and tips of lateral roots in SR cuttings 77 days after planting in T. chinensis. Compared to NR cuttings, SR cuttings had higher expression of polyphenoloxidase (PPO), catalase (CAT), peroxidase (POD), ascorbate peroxidase (APOX), glutathione reductase (GR), and superoxide dismutase (SOD) in the adventitious roots and basal portion of the rooted cuttings 77 days after planting. In the basal portion of cuttings, the content of thiobarbituric acid reactive substances (TBARS) and total phenols were decreased and the content of antioxidants was increased, but they did not changed in the needles of cuttings during planting. Analysis of microRNAs by quantitative realtime PCR demonstrated that expression of miR162, miR408, and miR857 increases in the basal portion of cuttings, but not in the stem portion of cuttings, 77 days after planting. Expression of miR408 and miR857 were also increased in the needles of cuttings 77 days after planting. Changes of these antioxidative enzymes and microRNAs are associated with the rooting features of T. chinensis var. mairei cuttings. Our research revealed that the levels and changes in endogenous factors affecting the rooting of cuttings differ between easy-to-root and recalcitrant-to-root T. chinensis. The individual features of antioxidative enzymes and microRNAs would benefit the development of efficient propagation methods for cuttings of T. chinensis.
Acknowledgements
We are grateful to the university research instrumentation facility for providing necessary experiment set up. The authors are grateful to Dr. W. Thompson, Dr. P. Bradshaw, and Dr. M. Page for their critical reading and suggestions during the preparation of this manuscript. This work was supported by a grant from the National Natural Science Foundation of China (31270740).
References
- 1.M E Stevens, K E Woeste, Pijut P M. (2018) Localized gene expression changes during adventitious root formation in black walnut (Juglans nigra L.), doi: 10.1093/treephys/tpx175. Tree physiology.
- 2.Herrmann S, Recht S, Boenn M, Feldhahn L, Angay O et al. (2015) Endogenous rhythmic growth in oak trees is regulated by internal clocks rather than resource availability. , Journal of experimental botany 66, 7113-7127.
- 3.Abu-Abied M, Szwerdszarf D, Mordehaev I, Yaniv Y, Levinkron S et al. (2014) Gene expression profiling in juvenile and mature cuttings of Eucalyptus grandis reveals the importance of microtubule remodeling during adventitious root formation. , BMC genomics 15, 826.
- 4.B E Llorente, Larraburu E E. (2013) In vitro propagation of fraser photinia using Azospirillum-mediated root development, Methods in molecular biology,11013,245-258.
- 5.Zhang Q, Liu H, Sun G, I W Wilson, Wu J et al. (2015) Baseline survey of root-associated microbes of Taxus chinensis (Pilger) Rehd. , PloS one 10, 0123026.
- 6.Syklowska-Baranek K, Pietrosiuk A, Kokoszka A, Furmanowa M. (2009) Enhancement of taxane production in hairy root culture of Taxus x media var. , Hicksii, Journal of plant physiology 166, 1950-1954.
- 7.Skorupinska-Tudek K, Pytelewska A, Zelman-Femiak M, Mikoszewski J, Olszowska O et al. (2007) In vitro plant tissue cultures accumulate polyisoprenoid alcohols. , Acta biochimica Polonica 54, 847-852.
- 8.Fattorini L, Veloccia A, F Della Rovere, D’Angeli S, Falasca G et al. (2017) Indole-3-butyric acid promotes adventitious rooting in Arabidopsis thaliana thin cell layers by conversion into indole-3-acetic acid and stimulation of anthranilate synthase activity. , BMC plant biology 17, 121.
- 9.Yan Y H, J L Li, X Q Zhang, W Y Yang, Wan Y et al. (2014) Effect of naphthalene acetic acid on adventitious root development and associated physiological changes in stem cutting of Hemarthria compressa. , PloS one 9, 90700.
- 10.Katayama M, Masui Y, Kageyama E, Kawabata Y, Kanayama K. (2008) Synthesis and biological activities of 4-trifluoromethylindole-3-acetic acid: a new fluorinated indole auxin. , Bioscience, biotechnology, and biochemistry 72, 2025-2033.
- 11.G C Pagnussat, M L Lanteri, Lamattina L. (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process, Plant physiology. 132, 1241-1248.
- 12.C L Ribeiro, C M Silva, Drost D R, Novaes E, C R Novaes et al. (2016) Integration of genetic, genomic and transcriptomic information identifies putative regulators of adventitious root formation in Populus. , BMC plant biology 16, 66.
- 13.T D Salla, R da Silva, L V Astarita, E R Santarem. (2014) Streptomyces rhizobacteria modulate the secondary metabolism of Eucalyptus plants, Plant physiology and biochemistry :. , PPB 85, 14-20.
- 14.B E Sample, Lowe J, Seeley P, Markin M, McCarthy C et al. (2015) Depth of the biologically active zone in upland habitats at the Hanford Site, Washington: Implications for remediation and ecological risk management, Integrated environmental assessment and management. 11, 150-160.
- 15.Veld WA Man In 't, Rosendahl K C, PC van Rijswick, Meffert J P, Westenberg M et al.FA van Kuik (2015)Phytophthora terminalis sp. nov. and Phytophthora occultans sp. nov., two invasive pathogens of ornamental plants in Europe. 107-54.
- 16.T Y Dou, H W Luan, X B Liu, S Y Li, X F Du et al. (2015) Enzymatic hydrolysis of 7-xylosyltaxanes by an extracellular xylosidase from Cellulosimicrobium cellulans. , Biotechnology letters 37, 1905-1910.
- 17.Mazzio E, Badisa R, Mack N, Deiab S, K F Soliman. (2014) High throughput screening of natural products for anti-mitotic effects in MDA-MB-231 human breast carcinoma cells. , Phytotherapy research : PTR 28, 856-867.
- 18.Jimenez J T, Sturdikova M, Brezova V, Svajdlenka E, Novotova M. (2012) Screening of mutant strain Streptomyces mediolani sp. AC37 for (-)-8-O-methyltetrangomycin production enhancement. , Journal of microbiology 50, 1014-1023.
- 19.Brunakova K, Kosuth J. (2009) Gene expression profiling in Taxus baccata L. seedlings and cell cultures, Methods in molecular biology. 547, 249-262.
- 20.Onrubia M, Pollier J, R Vanden Bossche, Goethals M, Gevaert K et al. (2014) Taximin, a conserved plant-specific peptide is involved in the modulation of plant-specialized metabolism. , Plant biotechnology journal 12, 971-983.
- 21.Rosangkima G, S B Prasad. (2004) Antitumour activity of some plants from Meghalaya and Mizoram against murine ascites Dalton's lymphoma, Indian journal of experimental biology. 42, 981-988.
- 22.Li Y, Yang J, Zhou X, Zhao W, Jian Z. (2015) Isolation and identification of a 10-deacetyl baccatin-III-producing endophyte from Taxus wallichiana, Applied biochemistry and biotechnology. 175, 2224-2231.
- 23.C Hao da, Ge G, Xiao P, Zhang Y, Yang L. (2011) The first insight into the tissue specific taxus transcriptome via Illumina second generation sequencing. , PloS one 6, 21220.
- 24.Quan J, Zhang S, Zhang C, Meng S, Zhao Z et al. (2014) Molecular cloning, characterization and expression analysis of the SAMS gene during adventitious root development in IBA-induced tetraploid black locust. , PloS one 9, 108709.
- 25.A I Sannazzaro, Echeverria M, E O Alberto, O A Ruiz, A B Menendez. (2007) Modulation of polyamine balance in Lotus glaber by salinity and arbuscular mycorrhiza, Plant physiology and biochemistry :. , PPB 45, 39-46.
- 26.Niemi K, Haggman H, Sarjala T. (2002) Effects of exogenous diamines on the interaction between ectomycorrhizal fungi and adventitious root formation in Scots pine in vitro. , Tree physiology 22, 373-381.
- 27.Tonon G, Kevers C, Gaspar T. (2001) Changes in polyamines, auxins and peroxidase activity during in vitro rooting of Fraxinus angustifolia shoots: an auxin-independent rooting model, Tree physiology. 21, 655-663.
- 28.Basiglini E, Pintore M, Forni C. (2018) Effects of treated industrial wastewaters and temperatures on growth and enzymatic activities of duckweed (Lemna minor L.), Ecotoxicology and environmental safety,153;54-59.
- 29.Spanic V, M Viljevac Vuletic, Abicic I, Marcek T. (2017) Early response of wheat antioxidant system with special reference to Fusarium head blight stress, Plant physiology and biochemistry : PPB. 115, 34-43.
- 30.Iqbal A, Wang T, Wu G, Tang W, Zhu C et al. (2017) Physiological and transcriptome analysis of heteromorphic leaves and hydrophilic roots in response to soil drying in desert Populus euphratica. , Scientific reports 7, 12188.
- 31.JJ Dragisic Maksimovic, Poledica M M, Radivojevic D D, Milivojevic J M. (2017) Enzymatic Profile of 'Willamette' Raspberry Leaf and Fruit Affected by Prohexadione-Ca and Young Canes Removal Treatments. , Journal of agricultural and food chemistry 65, 5034-5040.
- 32.Palanisamy S, A K Mandal. (2014) Susceptibility against grey blight disease-causing fungus Pestalotiopsis sp. in tea (Camellia sinensis (L.) O. Kuntze) cultivars is influenced by anti-oxidative enzymes, Applied biochemistry and biotechnology. 172, 216-223.
- 33.A R Soares, Ferrarese M de Lourdes Lucio, Siqueira-Soares R de Cassia, Marchiosi R, Finger-Teixeira A et al. (2011) The allelochemical L-DOPA increases melanin production and reduces reactive oxygen species in soybean roots. , Journal of chemical ecology 37, 891-898.
- 34.Montavon P, Kukic K R, Bortlik K. (2007) A simple method to measure effective catalase activities: optimization, validation, and application in green coffee. , Analytical biochemistry 360, 207-215.
- 35.Bosselut N, C Van Ghelder, Claverie M, Voisin R, J P Onesto et al. (2011) Agrobacterium rhizogenes-mediated transformation of Prunus as an alternative for gene functional analysis in hairy-roots and composite plants, Plant cell reports. 30, 1313-1326.
- 36.Trobec M, Stampar F, Veberic R, Osterc G. (2005) Fluctuations of different endogenous phenolic compounds and cinnamic acid in the first days of the rooting process of cherry rootstock 'GiSelA 5' leafy cuttings. , Journal of plant physiology 162, 589-597.
- 37.Esmenjaud D, Minot J C, Voisin R, Salesses G, Bonnet A. (1995) Effect of Cutting Age on the Resistance of Prunus cerasifera (Myrobalan Plum) to Meloidogyne arenaria. , Journal of nematology 27, 634-638.
- 38.Wilkins D, JJ Van Oosten, R T Besford. (1994) Effects of elevated CO(2) on growth and chloroplast proteins in Prunus avium. , Tree physiology 14, 769-779.
- 39.M K Kanwar, Poonam S Pal, Bhardwaj R. (2015) Involvement of Asada-Halliwell Pathway During Phytoremediation of Chromium (VI). in Brassica juncea L. Plants, International journal of phytoremediation 17, 1237-1243.
- 40.Sharma I, Pati P K, Bhardwaj R. (2010) Regulation of growth and antioxidant enzyme activities by 28-homobrassinolide in seedlings of Raphanus sativus L. under cadmium stress. , Indian journal of biochemistry & biophysics 47, 172-177.
- 41.Arora P, Bhardwaj R, M Kumar Kanwar. (2010) 24-epibrassinolide induced antioxidative defense system of Brassica juncea L. under Zn metal stress, Physiology and molecular biology of plants : an international journal of functional plant biology. 16, 285-293.
- 42.Bhatnagar-Mathur P, Devi M J, Vadez V, Sharma K K. (2009) Differential antioxidative responses in transgenic peanut bear no relationship to their superior transpiration efficiency under drought stress. , Journal of plant physiology 166, 1207-1217.
- 43.Stobrawa K, Lorenc-Plucinska G. (2008) Thresholds of heavy-metal toxicity in cuttings of European black poplar (Populus nigra L.) determined according to antioxidant status of fine roots and morphometrical disorders, The Science of the total environment. 390, 86-96.
- 44.Arican E, Gozukirmizi N. (2008) Effects of hyperbaric oxygenation on cultured barley embryos. , Acta biologica Hungarica 59, 453-464.
- 45.Tang W, T M Charles, R J Newton. (2005) Overexpression of the pepper transcription factor CaPF1 in transgenic Virginia pine (Pinus Virginiana Mill.) confers multiple stress tolerance and enhances organ growth, Plant molecular biology. 59, 603-617.
- 46.M V Salomon, Piccoli P, I Funes Pinter, W A Stirk, Kulkarni M et al. (2017) Bacteria and smoke-water extract improve growth and induce the synthesis of volatile defense mechanisms in Vitis vinifera L, Plant physiology and biochemistry : PPB. 120, 1-9.
- 47.P Y Chen, Y I Lee, B C Chen, K W Juang. (2013) Effects of calcium on rhizotoxicity and the accumulation and translocation of copper by grapevines, Plant physiology and biochemistry :. , PPB 73, 375-382.
- 48.P F Bert, Bordenave L, Donnart M, Hevin C, Ollat N et al. (2013) Mapping genetic loci for tolerance to lime-induced iron deficiency chlorosis in grapevine rootstocks (Vitis sp.), TAG. Theoretical and applied genetics. Theoretische und angewandte. , Genetik 126, 451-473.
- 49.Ksouri R, Debez A, Mahmoudi H, Ouerghi Z, Gharsalli M et al. (2007) Genotypic variability within Tunisian grapevine varieties (Vitis vinifera L.) facing bicarbonate-induced iron deficiency, Plant physiology and biochemistry :. , PPB 45, 315-322.
- 50.A R Khan, Ullah I, Waqas M, G S Park, A L Khan et al. (2017) Host plant growth promotion and cadmium detoxification in Solanum nigrum, mediated by endophytic fungi, Ecotoxicology and environmental safety,136;180-188.
- 51.P B Goud, M S Kachole. (2012) Antioxidant enzyme changes in neem, pigeonpea and mulberry leaves in two stages of maturity. , Plant signaling & behavior 7, 1258-1262.
- 52.Liang G, Ai Q, Yu D. (2015) Uncovering miRNAs involved in crosstalk between nutrient deficiencies in Arabidopsis, Scientific reports. 5, 11813.
- 53.Liang G, He H, Yu D. (2012) Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. , PloS one 7, 48951.
- 54.Yamasaki H, Hayashi M, Fukazawa M, Kobayashi Y, Shikanai T. (2009) SQUAMOSA Promoter Binding Protein-Like7 Is a Central Regulator for Copper Homeostasis in Arabidopsis, The Plant cell. 21, 347-361.
- 55.S E Abdel-Ghany, Pilon M. (2008) MicroRNA-mediated systemic down-regulation of copper protein expression in response to low copper availability in Arabidopsis. , The Journal of biological chemistry 283, 15932-15945.
- 56.Druege U, Franken P, Lischewski S, Ahkami A H, Zerche S et al. (2014) Transcriptomic analysis reveals ethylene as stimulator and auxin as regulator of adventitious root formation in petunia cuttings, Frontiers in plant science. 5, 494.
- 57.Ahkami A H, Melzer M, M R Ghaffari, Pollmann S, M Ghorbani Javid et al. (2013) Distribution of indole-3-acetic acid in Petunia hybrida shoot tip cuttings and relationship between auxin transport, carbohydrate metabolism and adventitious root formation. , Planta 238, 499-517.
- 58.Ghorecha V, Zheng Y, Liu L, Sunkar R, Krishnayya N S R. (2017) MicroRNA dynamics in a wild and cultivated species of Convolvulaceae exposed to drought stress, Physiology and molecular biology of plants : an international journal of functional plant biology. 23, 291-300.
- 59.Chen Q, Li M, Zhang Z, Tie W, Chen X et al. (2017) Integrated mRNA and microRNA analysis identifies genes and small miRNA molecules associated with transcriptional and post-transcriptional-level responses to both drought stress and re-watering treatment in tobacco. , BMC genomics 18, 62.
- 60.Roy S, A M Tripathi, Yadav A, Mishra P, C S Nautiyal. (2016) Identification and Expression Analyses of miRNAs from Two Contrasting Flower Color Cultivars of Canna by Deep Sequencing. , PloS one 11, 0147499.
- 61.Shao F, Qiu D, Lu S. (2015) Comparative analysis of the Dicer-like gene family reveals loss of miR162 target site. in SmDCL1 from Salvia miltiorrhiza, Scientific reports 5, 9891.
- 62.Fu W, Zhu P, Wang C, Huang K, Du Z et al. (2015) A highly sensitive and specific method for the screening detection of genetically modified organisms based on digital PCR without pretreatment. , Scientific reports 5, 12715.
- 63.Gielen H, Remans T, Vangronsveld J, Cuypers A. (2016) Toxicity responses of Cu and Cd: the involvement of miRNAs and the transcription factor SPL7. , BMC plant biology 16, 145.
- 64.Zhao Y, Lin S, Qiu Z, Cao D, Wen J et al. (2015) . MicroRNA857 Is Involved in the Regulation of Secondary Growth of Vascular Tissues in Arabidopsis, Plant physiology 169-2539.
- 65.Hedayati V, Mousavi A, Razavi K, Cultrera N, Alagna F et al. (2015) Polymorphisms in the AOX2 gene are associated with the rooting ability of olive cuttings, Plant cell reports. 34, 1151-1164.
- 66.Girijashankar V. (2012) In vitro regeneration of Eucalyptus camaldulensis, Physiology and molecular biology of plants : an international journal of functional plant biology. 18, 79-87.
- 67.Tang W, R J Newton, Li C, T M Charles. (2007) Enhanced stress tolerance in transgenic pine expressing the pepper CaPF1 gene is associated with the polyamine biosynthesis, Plant cell reports. 26, 115-124.
- 68.Tang W, R J Newton. (2005) Polyamines promote root elongation and growth by increasing root cell division in regenerated Virginia pine (Pinus virginiana Mill.) plantlets, Plant cell reports. 24, 581-589.
- 69.Arias M, Carbonell J, Agusti M. (2005) Endogenous free polyamines and their role in fruit set of low and high parthenocarpic ability citrus cultivars. , Journal of plant physiology 162, 845-853.
- 70.N T Dutra, Silveira V, Azevedo I G de, L R Gomes-Neto, A R Facanha et al. (2013) Polyamines affect the cellular growth and structure of pro-embryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines. , Physiologia plantarum 148, 121-132.
- 71.B C Chen, P C Ho, K W Juang. (2013) Alleviation effects of magnesium on copper toxicity and accumulation in grapevine roots evaluated with biotic ligand models. , Ecotoxicology 22, 174-183.
- 72.K W Juang, Y I Lee, H Y Lai, C H Wang, B C Chen. (2012) Copper accumulation, translocation, and toxic effects in grapevine cuttings, Environmental science and pollution research international. 19, 1315-1322.
- 73.Kose C, Erdal S, Kaya O, Atici O. (2011) Comparative evaluation of oxidative enzyme activities during adventitious rooting in the cuttings of grapevine rootstocks. , Journal of the science of food and agriculture 91, 738-741.
- 74.Katayama M. (2000) Synthesis and biological activities of 4-chloroindole-3-acetic acid and its esters. , Bioscience, biotechnology, and biochemistry 64, 808-815.
- 75.A C Nordstrom, F A Jacobs, Eliasson L. (1991) Effect of Exogenous Indole-3-Acetic Acid and Indole-3-Butyric Acid on Internal Levels of the Respective Auxins and Their Conjugation with Aspartic Acid during Adventitious Root Formation in Pea Cuttings, Plant physiology. 96, 856-861.
- 76.Fujihara S, Yoneyama T. (2001) Endogenous levels of polyamines in the organs of cucumber plant (Cucumis sativus) and factors affecting leaf polyamine contents, Physiologia plantarum. 113, 416-423.
- 77.Noriega X, Burgos B, F J Perez. (2007) Short day-photoperiod triggers and low temperatures increase expression of peroxidase RNA transcripts and basic peroxidase isoenzyme activity in grapevine buds. , Phytochemistry 68, 1376-1383.
- 78.Mou D, Yao Y, Yang Y, Zhang Y, Tian C et al. (2011) Plant high tolerance to excess manganese related with root growth, manganese distribution and antioxidative enzyme activity in three grape cultivars, Ecotoxicology and environmental safety. 74, 776-786.
- 79.CT da Costa, MR de Almeida, C M Ruedell, Schwambach J, F S Maraschin et al. (2013) When stress and development go hand in hand: main hormonal controls of adventitious rooting in cuttings, Frontiers in plant science. 4, 133.
- 80.E Santos Macedo, H G Cardoso, Hernandez A, Peixe A A, Polidoros A et al. (2009) Physiologic responses and gene diversity indicate olive alternative oxidase as a potential source for markers involved in efficient adventitious root induction, Physiologia plantarum. 137, 532-552.
- 81.Krahulcova A, Travnicek P, Krahulec F, Rejmanek M. (2017) Small genomes and large seeds: chromosome numbers, genome size and seed mass in diploid Aesculus species (Sapindaceae),Annals of botany,119;957-964.
- 82.Druege U, Franken P, M R Hajirezaei. (2016) Plant Hormone Homeostasis, Signaling, and Function during Adventitious Root Formation in Cuttings, Frontiers in plant science. 7, 381.
- 83.Ishida T, Adachi S, Yoshimura M, Shimizu K, Umeda M et al. (2010) Auxin modulates the transition from the mitotic cycle to the endocycle in Arabidopsis. , Development 137, 63-71.
- 84.Boudolf V, Lammens T, Boruc J, J Van Leene, Daele H Van Den et al. (2009) CDKB1;1 forms a functional complex with CYCA2;3 to suppress endocycle onset. , Plant physiology 150, 1482-1493.
- 85.Breuer C, Ishida T, Sugimoto K. (2010) Developmental control of endocycles and cell growth in plants, Current opinion in plant biology. 13, 654-660.
- 86.Wildwater M, Campilho A, J M Perez-Perez, Heidstra R, Blilou I et al. (2005) The RETINOBLASTOMA-RELATED gene regulates stem cell maintenance in Arabidopsis roots. , Cell 123, 1337-1349.
- 87.Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I et al. (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. , Nature 433, 39-44.
- 88.Vuosku J, Suorsa M, Ruottinen M, Sutela S, Muilu-Makela R et al. (2012) Polyamine metabolism during exponential growth transition in Scots pine embryogenic cell culture. , Tree physiology 32, 1274-1287.
- 89.Sarjala T, Niemi K, Haggman H. (2010) Mycorrhiza formation is not needed for early growth induction and growth-related changes in polyamines. in Scots pine seedlings in vitro, Plant physiology and biochemistry : PPB 48, 596-601.
- 90.Hajyzadeh M, Turktas M, Khawar K M, T. (2015) Unver, miR408 overexpression causes increased drought toleranceinchickpea,Gene,555;186-193.
- 91.Jovanovic Z, Stanisavljevic N, Mikic A, Radovic S, Maksimovic V. (2014) . Water deficit down-regulates miR398 and miR408 in pea (Pisum sativum L.), Plant physiology and biochemistry : PPB 83, 26-31.
- 92.R D Mutum, S C Balyan, Kansal S, Agarwal P, Kumar S et al. (2013) Evolution of variety-specific regulatory schema for expression of osa-miR408 in indica rice varieties under drought stress. , The FEBS journal 280, 1717-1730.
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
Yamei Wang, Xueke Chen, Jingguang Chen - Plant Science (2024) Semantic Scholar