Treatment of a Severe Pediatric Lyell Syndrome with Amniotic Membrane: Case Report and Histological Findings
Abstract
Background:
Lyell Syndrome (TEN, Toxic epidermal necrolysis) represents a medical emergency particularly in pediatric patients in whom the massive skin damage can quickly lead to multi-organ dysfunction and death. Prompt restoration of the physiologic mucosal/cutaneous barrier is mandatory. The use of amniotic membranes has been described in the treatment of ophthalmic Lyell Syndrome, but its use has not yet been adopted for the management of larger cutaneous wounds.
Study Hypothesis:
Here we report the use of amniotic membranes in a pediatric case of severe Lyell Syndrome with complete skin surface, ocular and mucosal involvement with life threating presentation.
Methods:
A 7-year old female was admitted to our Burn Centre for severe cutaneous/mucosal exfoliation (100% Total body surface area, TBSA) as a result of an adverse reaction to ibuprofen administration. Supportive fluid administration, cardiac-pulmonary assistance and pain management were complemented by serial grafting of amniotic membranes on all affected areas to provide coverage of the exfoliated skin/mucosa. Biopsies were obtained to monitor histological skin changes.
Results:
The patient showed an excellent response to amniotic membrane treatment, with rapid restoration of mucosal and cutaneous layers in the grafted areas. This resulted in a decreased need for dressing changes, avoidance of additional surgeries and a reduced dependence on supportive therapy. Lower pain levels than usually expected led to a reduced need for narcotic pain medications and allowed for early physical rehabilitation and a short hospital stay. Histology confirmed evidence of topical immune-modulation in treated areas (reduction of inflammatory infiltrate).
Conclusion:
As we tested in numerously TEN and burn pediatric injuries Amniotic membranes with their regenerative and immune-modulatory effects may represent an life saving treatment even in the worst cases of pediatric Lyell syndrome.
Author Contributions
Academic Editor: Jun Wan, Department of Medical and Molecular Genetics, Indiana University School of Medicine, Unıted states.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2018 B.Azzena, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Lyell syndrome (or toxic epidermal necrolysis, TEN) is a rare life-threatening severe cutaneous adverse reaction to drugs 1 . It's a disease with common causes and mechanisms with SJS ( Stevens- Johnson syndrome) but the principal difference is the extent of detachment, limited in SJS ( usually from 10% to 30% of TBSA) and more widespread in TEN ( over than 30% TBSA) 2 This acute inflammatory systemic condition results in extensive epidermal sloughing with an associated mortality of 25 to 75%. The overall incidence of TEN is rare, estimated to be at 0.4 to 1.2 cases per million person-years and can occur in all age groups 3 The reported incidence of Lyell in children is lower than in adults 4. For the survivors the devastating sequelae of mucosal involvement, especially eye involvement, are a common treatment challenge 5 . The conjunctiva are the most-commonly affected mucosa surface, with an involvement rate ranging from 69% to 81% in Stevens-Johnson syndrome and 50% to 67% in Lyell syndrome 6, 7. Many common drugs can cause TEN, in fact more than 200 medications have been implicated, including sulphonamide antibiotics, penicillins, allopurinol and oxicam 8 In the majority of pediatric cases, drugs were implicated as the most common cause of TEN followed by viral and bacterial infections 9. TEN is a delayed-type hypersensitivity reaction (or type IV reaction); despite still-unclear pathophysiology, a genetic pattern of susceptibility has been purposed 10 . Several treatment modalities have been proposed with variable results, but there is a paucity of high-quality evidence upon which to base treatment of this rare disease, especially in children. For these reasons, the management and systemic treatment of these syndromes have not been standardised. The only treatment that has been proven to improve survival is the rapid withdrawal of the suspected offending drugs and an optimal supportive therapy. Use of systemic corticosteroid, antibiotic administration and immunomodulatory drugs remain controversial, especially if we consider large studies where are reported TEN cases onset due to all of these treatment 1. Prompt and adequate management of affected mucosal and cutaneous areas is important , especially when more sensitive body regions are involved (face, peri-ocular and peri-oral areas, hands, external genitalia) as well as when a TBSA higher than 30% is involved. As such, treatment by Burn Teams has been shown to improve outcomes. The ideal topical treatment remains controversial, particularly for severe cases.
Case Report
A 7 -year-old female child was admitted to our Burn Centre for severe cutaneous and mucosal exfoliation (100% total body surface area, TBSA) as a result of an adverse reaction to ibuprofen administration. The patient was otherwise healthy and with no congenital abnormalities, no previous systemic diseases, and no history of allergies or previous traumas [Figure 1]. The ibuprofen was the only drug provide to the patient to treat fever comparison and the onset of the lesions started rapidly from 8 hour after the assumption. The dose was 400 mg of ibuprofen and it has seems that was the first time that such drug was administered to the patient. Mucosal and eye involvement was present. Emergency care provided included appropriate cleaning of the lesions, anesthesiologist and endoscopic evaluation for airway evaluation and to assist in pain control. Pediatric and ophthalmologist evaluation were also performed. Because the severe drug reaction, no other systemic treatments were administered. The skin was covered with vaseline gauze and adequate fluid support and early enteral nutrition were provided. After 24 hours, under general anesthesia, wounds were treated with vaseline gauze and vitamin E spray solution ( VEA® oil). A skin biopsy was obtained at the axillary region, confirming TEN diagnosis by clearly visualization of necrotic process and slough of the basal layer. Afterwards, wound care under general anesthesia was performed every two days for one week, with poor skin healing observed. Therefore, a surgical procedure was planned with careful debridement of the necrotic epidermal layer and coverage with amniotic membrane (AM) provided by our human tissue bank on day 8 after admission [Figure 2]. AM it’s provided in measure from 3x3 cm (ocular patch) till 10x15 cm for wide area coverage. In the present case over than ten maximal patch were used in order to cover the widest lesion area as possible, particularly the anterior trunk, arms , hands and legs and face. The overlapping areas of AM pieces were sewed where necessary and immobilization was obtained by administration of nebulized tissue glue (ARTISS ® , Baxter-USA) and also was covered with a silicone sheet medication to improve its stabilization(Mepitel®, Molnlycke-Sweden). Joint areas were not covered due the high risk of AM loss during movements.AM treatment was also employed for conjunctival lesion by its softly deposition on ocular surface by using the proper ocular patch[Figure 3]. No other medications were administered for the subsequent three days, showing a good control of fluid loss, pain, good clinical outcome, and absence of infection. On day 14 after admission the patient underwent a complete medication and evaluation under anesthesia, which showed rejection of the AM and advanced re-epithelization of most areas (90% of TBSA restoring). A sample of rejected AM was submitted to histological analysis [Figure 4]. The AM specimen were taken and rinsed in phosphate buffered saline (PBS) and then fixed in 10% buffered formalin for general histology; 4% paraformaldehyde for immunohisto chemistry. In the meanwhile no further procedures were needed for the patient and complete healing (100% of TBSA) was achieved after 1 week and patient was discharged on day 24 after admission. At six months follow up, no scars were present with satisfactory cosmetic result and no mucosal or eye sequelae [Figure 5, Figure 6]. Testing the visual acuity no impairment were noted with complete restoring of conjunctival aspects.
Figure 1.Emergency care of Lyell onset. Skin lesions were involving 100% TBSA. Here we see the typical aspect at trunk and arms level.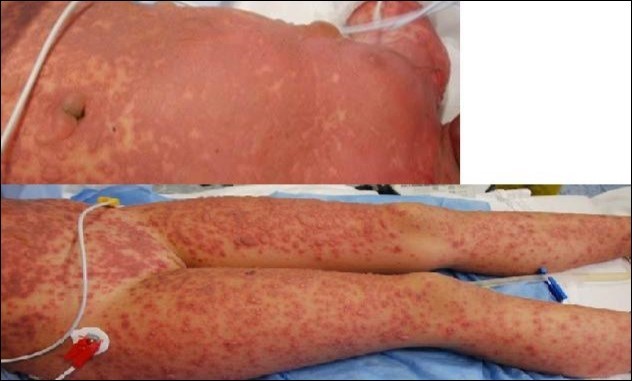
Figure 2.Application of Amniotic Membrane (AM) on chest level after surgical debridement.
Figure 3.Typical aspect of eye damage in Lyell syndrome. Please note application of Amniotic Membrane surrounding that area and it was also applied for eye restoration.
Figure 4.Aspect of Amniotic Membrane at 7 day after surgery with skin healing. A part of AM was retain for histological consideration.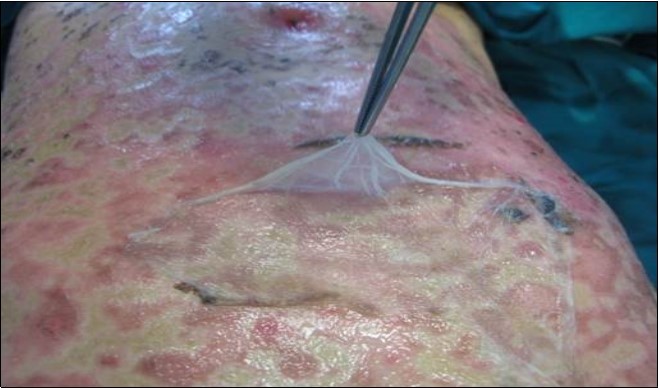
Figure 5.Six months follow up . Here we note trunk and neck with no scar evidence. Skin hyperemia and dyschromia are still present but with satisfactory cosmetic result and progressive regression.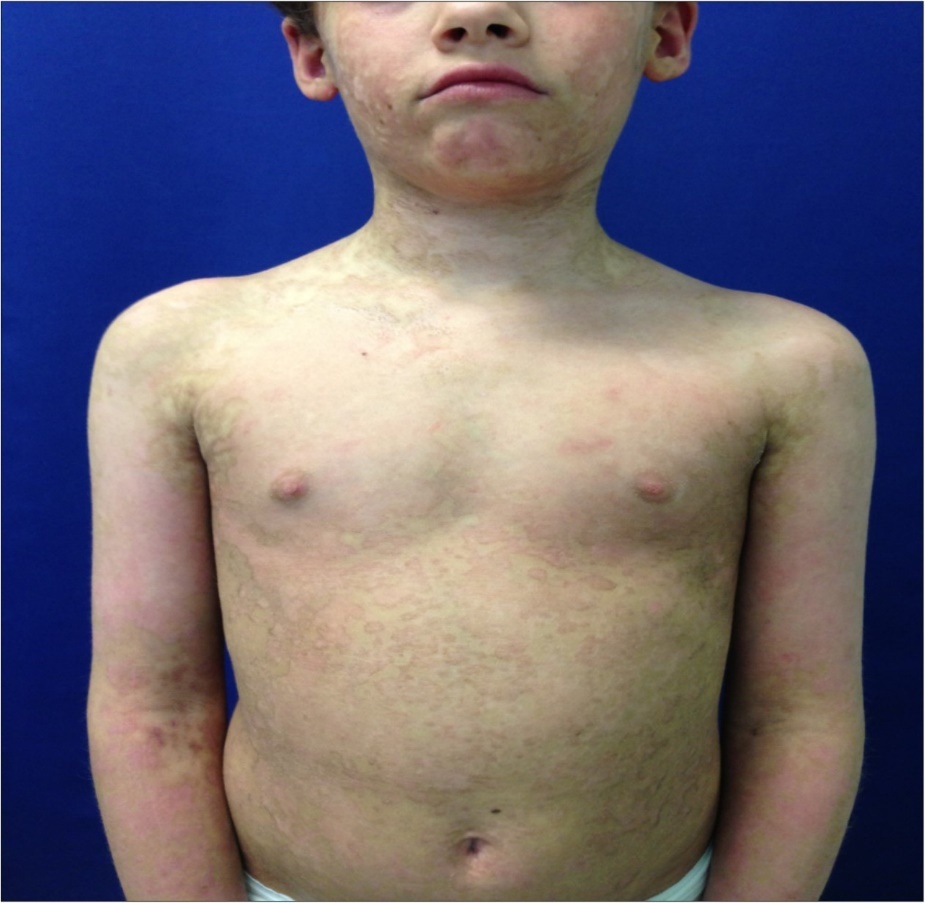
Figure 6.Particular of the complete eye mucosal healing without damage.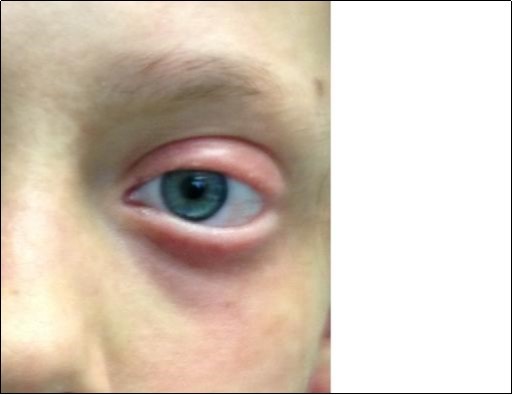
Discussion
Toxic epidermal necrolysis (TEN), also known as Lyell's syndrome, is a rare, life-threatening disease, characterized by extensive epidermal detachment, erosion of mucous membranes and severe systemic symptoms. It carries a high mortality rate due to complications of systemic infection and multiple organ failure , with reports ranging from 25% to 70% 11 . The early referral to a Burn Centre and advancements in supporting treatment have provided a reduction of mortality to 25-30% for TEN 12. The pediatric mortality rate has been reported as lower than in adults 13. A particularly strong association with TEN was reported for sulphonamides, allopurinol, anticonvulsants and non-steroidal anti-inflammatory drugs of the oxicam group, aminopenicillins, cephalosporins and quinolones 14 . Ibuprofen is linked to a worsening of the disease progression and incidence of more complications 15, 16 . This abnormal reaction seems to have a genetic correlation with drug hypersensitivity 17. Pathogenic mechanism of the skin loss is caused by the necrosis of keratinocytes following apoptosis due to an abnormal activation of CD8+ T lymphocytes 18. The exposure to a drug induces their maturation into cytotoxic T lymphocytes by the MHC I complex 5 stimulation or an hapten/hapten abnormal recognition mechanism 19 These points highlight the importance of identifying and discontinuing the inciting agent, if it is all possible20. Local treatment include surgical debridement with placement of biological or biosynthetic dressings, or topical antibiotic ointments.
For extensive lesions, intensive care is also recommended 21. The administration of intravenous immunoglobulins, corticosteroid or immunosuppressant as cyclosporine, cyclophosphamide or thalidomide have been described, but remain controversial 22. Greater risk of poor outcome in cases of prolonged use is well known 23. In our opinion, because this condition is particularly linked to drug administration, treatment must aimed to obtain an efficient and fast healing without use of superfluous drugs. For that reason we have focused our attention in regenerative skin properties of the amniotic membrane (AM) for its clinical application in severe TEN resolution 24. The AM is a thin translucent membrane which thickness varies from 0.02 to 0.05 mm. It first surrounds the embryo, later the foetus, and it takes origin from the innermost trophoblastic cells during the embryo development . It has no nerves, muscles or lymph vessels inside and it provides the embryo a protective barriers against infections and external pressures 25. AM has anti-inflammatory, anti-bacterial, anti-viral and immunological characteristics, as well as anti-angiogenic and pro-apoptotic features 26. AM is a promoter of epithelialization and is a non-tumorigenic tissue and its use has no ethical problems, making it a very useful tool for plastic surgery purposes 27 . Particularly important for the resolution of TEN lesions are the anti-inflammatory and epithelization-promoting properties of AM. In vitro was demonstrated the secretion of soluble proteins, such as tissue inhibitor metalloproteinases (TIMP-1,2,4), IL1-receptor antagonist, IL10, exerting the block of inflammatory response 28. Moreover epithelization induced by amnion, with cells containing epidermal growth factor (EGF), keratinocyte growth factor (KGF), keratinocyte growth factor receptor (KGFR), hepatocyte growth factor (HGF), and hepatocyte growth factor receptor (HGFR) identified. Recently the use of amniotic membranes has been successfully adopted in the treatment of specific mucosal lesions such as ophthalmic involvement in Stevens-Johnson disease, leading to a prompt and scar-free healing, apparently due to immuno-modulatory and pro-regenerative abilities 29 . In our experience from 2013 to 2016 we have successfully treated with AM a case series of 31 patients ( 26 pediatric) affected by scald burns (24 patients) and TEN syndrome (7 patients with an increasing use and experience among every year. The average of TBSA was 40% (17 to 61%) and in the TEN cases the ocular involvement was detached in 4 patients, no one pediatric 2. The AM we used it's fresh, controlled by infective agents according to National Guide Line for Transplantation , constituted by a single epithelial layer on a basal membrane and an avascular stroma; the chorion component are linked to a metal nipper to show to the surgeon the correct side for its application. The presence of near Tissue Bank with rapid disposition of such tissue are fundamental , particularly in case of eye or mucosal lesion for rapid healing. In the reported case we have firstly faced up a pediatric TEN episode more than 80% of TBSA with contemporary eye severe involvement. Based on our positive case series experience the use of AM for the total-body coverage of these severe TEN patient and related histological aspects are herein first described as an useful and saving life treatment. Compared to standard treatment AM provided a rapid and efficient epithelization with fluid loss control, infectious barrier and pain relief, allowing reduced drug support. Also, prompt healing of mucosal and eye surfaces played a fundamental role in restarting oral nutrition and providing a psychological improvement. Anti inflammatory action of the AM led to pain levels lower than usually expected in a similar situation reducing need for narcotic medications. The pain level was asses with the pain visual analog scale for pain (VAS) with a reduction from value 8 (intense pain) in the first week where we performed medication under general anesthesia every two days to value 2 (mild pain) after AM application. Similar value are detected in our experience in pediatric patient by using several non biological dressing as biobrane®, Mepilex® and Suprathel® but their absence of biological effect as described with AM and the low control of infection lead us to prefer AM as treatment in such severe cases. The non biological dressing still remain useful as coverage for AM application to protect it from accidental removal, friction damage and to maintain an ideal fluid control to prevent its exsiccation allowing to express all its properties. Antimicrobial effect of AM at last provide also the reduction of needed medication, passing from four to two per week , with another good result on reduction of overall pain that are related to post medication period. Reduction of pain symptoms allowed also an early mobilization of the patient that are fundamental on 100% TBSA patient to avoid worsening of back lesions. Complete recovery of independence in daily tasks and ambulation was obtained in 21 days: total hospitalization was reduced to 24 days, in comparison to our previous experience in similar case with medium period over than 40 days. Scar onset were not observed after three months supporting the theories of the scar prevention due to the inflammatory modulation provided by the AM, whereas similar injuries commonly shows initial onset of hypertrophic scar due to high inflammatory response after 4-5 weeks . Histological findings demonstrate that the native AM show a double layer with avascular stroma containing epithelial cell and on basement membrane several cells that were positively marked as CD34+ at immunochemistry staining, confirming them as hematopoietic progenitor stem cell [Figure 7]. Biopsy obtained by AM removal after 7 days show the conservation of its cytological architecture with regular host cell invasion without inflammatory response, and an attempt of pluristatification of epithelial cell [Figure 8]. The mean of such observation are unclear and we could not provide a complete explanation of the observed phenomenon, even if the most probable biologic mechanism are the AM role as an active scaffold for re epithelization process. Important aspects of such role seemed to be the bilayer structure and the presence of growth factor, epithelial membrane surface signals for homing process of circulating cells as also hematopoietic and stromal stem cell (hAMSc: human amniotic mesenchymal stem cell).
Figure 7.Immunochemistry findings on a native Amniotic Membrane retain before application on damaged skin. It’s possible to evaluate its structure with double layer with avascular stroma . The blue marked cell are positively to CD34+ stain as hematopoietic stem cell progenitor. Scale bar 1MM/0.01 mm.
Figure 8.Amniotic Membrane after remove in advanced epithelization. Please note conservation of its cytological pattern : epithelial layer, thick basement membrane and vascular connective tissue layer. No particular inflammatory response was evident. Scale bar 1MM/0.01 mm.
References
- 1.Mockenhaupt M, Viboud C, Dunant A, Naldi L, Halevy S et al. (2008) Steven Johnson syndrome and toxic epidermal necrolysis: assessment of medication risks with emphasis on recently marketed drugs. The Euro- SCAR-study. , J Invest Dermatol 128, 35-44.
- 2.Bastuji-Garin S, Rzany B, R S Stern, N H Shear, Naldi L et al. (1993) A clinical classification of cases of toxic epidermal necrolysis, Stevens–Johnson syndrome and erythema multiforme. , Arch Dermatol 129, 92-96.
- 3.Azzena B, Voltan A.A case of toxic epidermal necrolysis (TEN) with severe chronic ocular complications in a healthy 46-years-old woman Ann Burns Fire Disasters.2010Jun30. 23(2), 81-7.
- 4.Mockenhaupt M, Schopf E. (1996) Epidemiology of drug-induced severe skin react Semin Cutan Med Surg. 15, 236-43.
- 5.Power W J, Ghoraishi M, Merayo-Lloves J, Neves R A, Foster C S. (1995) Analysis of the acute ophthalmic manifestations of the erythema multiforme/ Stevens-Johnson syndrome/toxic epidermal necrolysis disease spectrum. , Ophthalmology 102(11), 1669-1676.
- 6.KC Ciralsky JB Sippel. (2013) Prompt versus delayed amniotic membrane application in a patient with acute Stevens-Johnson syndrome. , Clin Ophthalmol 7, 1031-4.
- 7.Y Kato, Hara H, Inagaki N, Nakamura M.Stevens-Johnson syndrome and toxic epidermal necrolysis: The Food and Drug Administration adverse event reporting system,2004-2013. Allergol Int.2015Jul. 64(3), 277-9.
- 8.Belver M T, Michavila A, Bobolea I, Feito M, Bellón T et al.Severe delayed skin reactions related to drugs in the paediatric age group: A review of the subject by way of three cases (Stevens-Johnson syndrome, toxic epidermal necrolysis and DRESS). Allergol Immunopathol (Madr).2015Jun15.
- 9.Kunimi Y, Hirata Y, Aihara M, Yamane Y, Ikezawa Z. (2011) Statistical analysis of StevenseJohnson syndrome caused by Mycoplasma pneumonia infection in Japan. , Allergol Int 60, 525-32.
- 10.Kaniwa N, Saito Y, Aihara M, Matsunaga K, Tohkin M et al. (2008) HLA-B locus in Japanese patients with anti-epileptics and allopurinol-related Stevens Johnson syndrome and toxic epidermal necrolysis. , Pharmacogenomics 9, 1617-22.
- 11.YL Heng YK Lim.Cutaneous adverse drug reactions in the elderly Curr Opin Allergy Clin Immunol.2015Aug. 15(4), 300-7.
- 12.McGee T, Munster A. (1998) Toxic epidermal necrolysis syndrome: mortality rate reduced with early referral to regional burn center. Plast Reconstr Surg. 102, 1018-1022.
- 13.Del Pozzo-Magana BR, Lazo-Langner A, Carleton B. (2011) A systematic review of treatment of drug-induced Stevens-Johnson syndrome and toxic epidermal necrolysis in children. , J Popul Ther Clin Pharmacol 18, 121-33.
- 14.Ferrándiz-Pulido C, García-Fernández D, Domínguez-Sampedro P. (2011) Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a review of the experience with paediatric patients in a University Hos¬pital. , J Eur Acad Dermatol Venereol 25, 1153-1159.
- 15.Roujeau J C, Kelly J P, Naldi L. (1995) Medication use and the risk of Stevens-Johnson syndrome or toxic epidermal necrolysis. , N Engl J Med 333, 1600-1607.
- 16.Dore J, Salisbury R E. (2007) Morbidity and mortality of mucocutaneous diseases in the pediatric population at a tertiary care center. , J Burn Care Res 28, 865-870.
- 17.Forman R, Koren G, Shear N H. (2002) Erythema multiforme, Stevens-Johnson syndrome and toxic epidermal necrolysis in children: a review of 10 years’ experience. , Drug Saf 25, 965-972.
- 18.Schwartz R A, McDonough P H, Lee B W. (2013) Toxic epidermal necrolysis: Part I. Introduction, history, classification, clinical features, systemic manifesta¬tions, etiology, and immunopathogenesis. , J Am Acad Dermatol
- 19.Paul C, Wolkenstein P, Adle H, Wechsler J, Garchon H J et al. (1996) Apoptosis as a mechanism of keratinocyte death in toxic epidermal necrolysis. , Br J Dermatol 134, 710-714.
- 20.Nassif A, Bensussan A, Boumsell L. (2004) Toxic epidermal necrolysis: ef¬fector cells are drug-specific cytotoxic T cells. , J Allergy Clin Immunol 114, 1209-1215.
- 21.Ferrandiz-Pulido C, Garcia-Patos V. (2013) A review of causes of Stevens-John¬son syndrome and toxic epidermal necrolysis in children. , Arch Dis Child 98, 998-1003.
- 22.Schwartz R A, McDonough P H, Lee B W. (2013) Toxic epidermal necrolysis: Part II. Prognosis, sequelae, diagnosis, differential diagnosis, prevention, and treatment. , J Am Acad Dermatol
- 23.Mockenhaupt M. (2011) The current understanding of Stevens−Johnson syn¬drome and toxic epidermal necrolysis. , Expert Rev Clin Immunol 7, 803-813.
- 24.Kardaun S H, Jonkman M F. (2007) Dexamethasone pulse therapy of Stevens Johnson Syndrom/TEN. Act Derm Venerol. 87, 144-148.
- 25.MJ Mamede AC Carvalho, Abrantes A M, Laranjo M, Maia C J, Botelho M F.Amniotic membrane: from structure and functions to clinical applications Cell Tissue Res.2012Aug. 349(2), 447-58.
- 26.Toda A, Okabe M, Yoshida T, Nikaido T.The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. , J Pharmacol Sci.2007Nov 105(3), 215-28.
- 27.Fairbairn N G, Randolph M A, Redmond R W.The clinical applications of human amnion in plastic surgery J Plast Reconstr Aesthet Surg.2014May. 67(5), 662-75.
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
J. R. Muñoz-Torres, Sidney B. Martínez-González, Alan D. Lozano-Luján, M. Martínez-Vázquez, Perla Velasco-Elizondo et al. - Frontiers in Bioengineering and Biotechnology (2023) Semantic Scholar
Frontiers in Bioengineering and Biotechnology (2023) OpenAlex
Frontiers in Bioengineering and Biotechnology (2023) Crossref