Analysis of Effects of Kale Powder Consumption among Subjects with Potential Metabolic Syndrome: A Prospective Single-Arm Clinical Study
Abstract
Objective:
The prospective intervention study was conducted to investigate the effects of kale powder (Kale juice mixed with water or milk) consumption on metabolic syndrome in subjects with potential metabolic syndrome.
Method:
In Arita-cho, 149 male and female subjects with potential metabolic syndrome were instructed to consume kale powder for 8 weeks, and its effects on blood pressure, HbA1c, BMI, abdominal circumference, and blood triglycerides, LDL-C, HDL-C, and fasting blood sugar levels were assessed. Additionally, the safety of kale powder was examined.
Results:
After the 8-week long intake of kale powder, a significant decrease was observed in laboratory and home test-based blood pressure, abdominal circumference, and levels of LDL-C, HDL-C, and fasting blood sugar. Additionally, a hypotensive effect was observed on conducting stratified analysis, in which patients with blood pressure-related diseases were excluded. Furthermore, no safety concerns were identified regarding kale powder.
Conclusion:
Kale powder had a beneficial effect to maintain optimal blood pressure, blood sugar, and abdominal circumference in subjects with potential metabolic syndrome. Additionally, a hypotensive effect was observed within the normal range in subjects without blood pressure-related diseases.
Author Contributions
Academic Editor: Sandeep Kumar, Emory University and Georgia Institute of Technology
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2016 Tomomi Ide, et al
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction:
Metabolic syndrome is a combination of disorders that includes abdominal obesity, hypertension, hyperglycemia, and disorder of lipid metabolism, which increases the risk for arteriosclerotic diseases such as cardiac disease and stroke. Based on the National Health and Nutrition Survey Japan, 2014 (by the Ministry of Health, Labour and Welfare), 16.3% of people aged 20 years or more are highly suspected to have metabolic syndrome. By including “those with potential metabolic syndrome,” the percentage increases to 30.7%, which reveals that about one third of individuals aged 20 years or more are highly suspected to have or develop metabolic syndrome.
The most important factor in treating suspected metabolic syndrome appropriately is to change to a healthier lifestyle, and the common health guidance includes exercise and diet therapy. However, many difficulties arise in continuation of the therapy. Supplemental intake of healthy foods as a part of the diet therapy is one of the easiest ways.
Therefore, routine intake of healthy foods to suppress the increase in the levels of blood pressure, glucose, and triglycerides is of great importance in preventing metabolic syndrome. Indeed, many studies have been published about the various functions of foods, such as the efficacy of indigestible dextrin or dietary fiber on postprandial glucose 1 and triglyceride levels2, and the efficacy of gamma-aminobutyric acid (GABA) on blood pressure 3, 4. These food components have specific health benefits.
Kale (Brassica oleracea var acephala, hagoromokanran in Japanese) used in this study is a cruciferous vegetable, which is originally from southern Europe; it has been cultivated as one of the edible vegetables since a long time ago. Currently, it is rarely consumed as an edible vegetable in Japan. However, kale is rich in β-carotene, vitamin C, calcium, and dietary fiber. Therefore, various age groups consume it as a useful food for comprehensive health management or for beauty purposes. It is generally consumed in the form of juice or dry powder for the daily supply of nutrients.
Additionally, kale is rich in various functional ingredients with diverse effects. Its effects on various lifestyle-related diseases have been recognized; for example, the effects of kale powder on blood cholesterol 5, blood sugar, and blood pressure 6 have been reported. However, only a few studies have been conducted regarding the effects on lifestyle-related diseases. Additionally, no study has currently been published for the effect of kale powder on metabolic syndrome.
In general, a randomized, placebo-controlled, and double blind study is conducted for foods with specified health use that require verification of effects. Although a similar study design is desirable for healthy foods, it is difficult to conduct an effective nutrient management intervention in many subjects. Furthermore, it is well known that various factors, such as mealtime and habits, influence the endpoint evaluation. To resolve these issues, a prospective intervention study is necessary under non-blinded conditions and randomization in an appropriate, large-scale population.
In this study, the prospective, single arm, intervention study of kale powder was conducted to investigate the effect on metabolic syndrome in male and female subjects with potential metabolic syndrome in Arita-cho, Saga in Japan.
Subjects and Methods
1. Study Subjects
This study included male and female subjects aged between 30 and 74 years who underwent health checkups in Arita-cho in Saga Prefecture, who were willing to participate in this study, who met the inclusion criteria of potential metabolic syndrome, and who did not meet any of the exclusion criteria.
The subjects were to meet any of the following inclusion criteria from 1 to 6: (1) abdominal circumference ≥ 85 cm in male subjects or ≥ 90 cm in female subjects, (2) triglycerides ≥ 150 mg/dL and/or HDL-C < 40 mg/dL, (3) systolic blood pressure (SBP) ≥ 120 mmHg and/or diastolic blood pressure (DBP) ≥ 80 mmHg, (4) fasting blood sugar ³110 mg/dL, (5) BMI ≥ 25, or (6) LDL-C ≥ 120 mg/dL.
The exclusion criteria were as follows: (1) regular intake of health supplements, including green juice; (2) a most recent laboratory-based test showed a significant deviation from the reference range for which the investigator deemed treatment was necessary; (3) definite diagnosis of atrial fibrillation; (4) history of serious heart, cerebrovascular, or kidney disease; (5) under treatment with warfarin potassium (inhibiting synthesis of vitamin K-dependent coagulation factors); (6) allergy to crucifers, including kale; (7) prior participation into another clinical research, including a clinical trial, within 2 months before start of this study; and (8) other patients whom the investigator or subinvestigator deemed inappropriate for the study subjects.
This study was conducted upon approval of the Ethical Review Board in Arita-cho (November 21, 2012) and the Institutional Review Board of HuBit genomix (November 1, 2012). The study is also registered UMIN Clinical Trials Registry System (UMIN000009408). The study subjects were thoroughly informed of contents of the study, and a written informed consent was obtained.
2. Food Used
In this study, kale powder containing 14 g of dry powder of kale leaves was used. The test food was individually packaged per 7 g of kale powder. A subject was instructed to take one package (7 g) of kale powder with about 100 to 150 mL of water or milk twice daily. Nutritional content per daily dose (7 g × 2 packages) was 42.8 kcal of energy, 2.76 g of protein, 0.58 to 0.90 g of lipids, and 2.88 to 4.66 g of carbohydrates.
3. Study Design
The study schedule is presented in Table 1. The subjects meeting the criteria for participation in the study were instructed to monitor diary intake for 4 weeks to accommodate to the study conditions before the intake of the test food (intervention period). Further, the subjects consumed the test food for 8 weeks. During the study period, the subjects were inhibited from excessive alcohol intake or exercise that greatly differed from daily habits, temperance in eating and excessive eating, and they were instructed to get enough sleep. Dinner on the day before the laboratory test was to be finished by 10 p.m., and the subjects were prohibited from eating and drinking (except for water) after dinner until the end of the laboratory test. Upon confirmation of fasting, the subjects underwent the laboratory test in the morning. On the day of the laboratory test, the subjects were prohibited from smoking until the end of the test.
Table 1. Study Schedule| Parameter | Screening period (4 weeks) | Intervention period (8 weeks) | ||
| Entry | Inclusion | week 0 | week 8 | |
| Explanation/informed consent | ● | |||
| Physical condition assessment | ● | ● | ● | |
| Inclusion | ● | |||
| Anthropometric measurement | ● | ● | ● | |
| Blood/urine test | ● | ● | ● | |
| Laboratory blood pressure | ● | ● | ● | |
| Test food intake | ||||
| Home blood pressure | ||||
| Diary recording | ||||
Assessment Method
Blood pressure was measured at home by each subject after awakening and before going to sleep every day using Omron’s auto blood pressure monitor HEM-8723. Other parameters were measured thrice at the time of entry, before the start of intake, and at week 8 of intake at the Welfare and Healthcare Center, where blood samples were also collected.
1) Efficacy Assessment
The efficacy variables included laboratory-based examination of blood pressure, home-based examination of blood pressure, blood lipids (triglycerides, LDL-cholesterol LDL and HDL-cholesterol HDL), fasting blood sugar, hemoglobin A1c (HbA1c), BMI, and abdominal circumference. They were measured at the beginning and the end of the study duration, and efficacy was assessed based on the changes.
2) Safety Assessment
(1) Laboratory test
The laboratory test included hematology (white blood cell count, red blood cell count, hemoglobin, hematocrit, mean corpuscular volume, mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration, and platelet count), blood biochemistry (fasting blood sugar, HbA1c, triglycerides, LDL-C, HDL-C, AST, ALT, γ-GTP, creatinine, and uric acid), and urinalysis (protein, glucose, urobilinogen, occult blood, ketone body, bilirubin, specific gravity, and pH).
(2) General measurement
Body weight, height, and abdominal circumference were measured.
(3) Adverse events
Adverse events were defined as abnormal changes in laboratory results and physical conditions for which causality with the test food could not be excluded by the investigator, and the number and the incidence of adverse events were calculated.
3) Statistical Analysis
Each measurement was presented as mean ± standard deviation. Changes between pre- and post-intake were compared using the paired Student’s t-test with a two-sided significance level of 0.05.
Results
1. Subjects
The subjects included in this study were managed as follows: 149 subjects were registered. Of these, 80 met the criteria of this study based on initial screening. Of these, 75 were included in the analysis and five were excluded owing to withdrawal from the study during the screening period. (The reason for exclusion is shown in Figure 1). Two subjects were further excluded from the efficacy analysis and 73 subjects were included. Further, 62 subjects were included in the blood pressure analysis, and 11 subjects were excluded; 71 subjects were included in the lipid analysis, and two subjects were excluded. For management, the data of triglyceride and fasting blood sugar levels were excluded for a subject who had consumed breakfast in the morning during enrollment (blood sampling within 150 minutes from meal). The flow chart of the subject management is shown in Figure
Figure 1.Subject Disposition. Flow chart of subjects detailing number withdrawing and excluding from the study, analysis.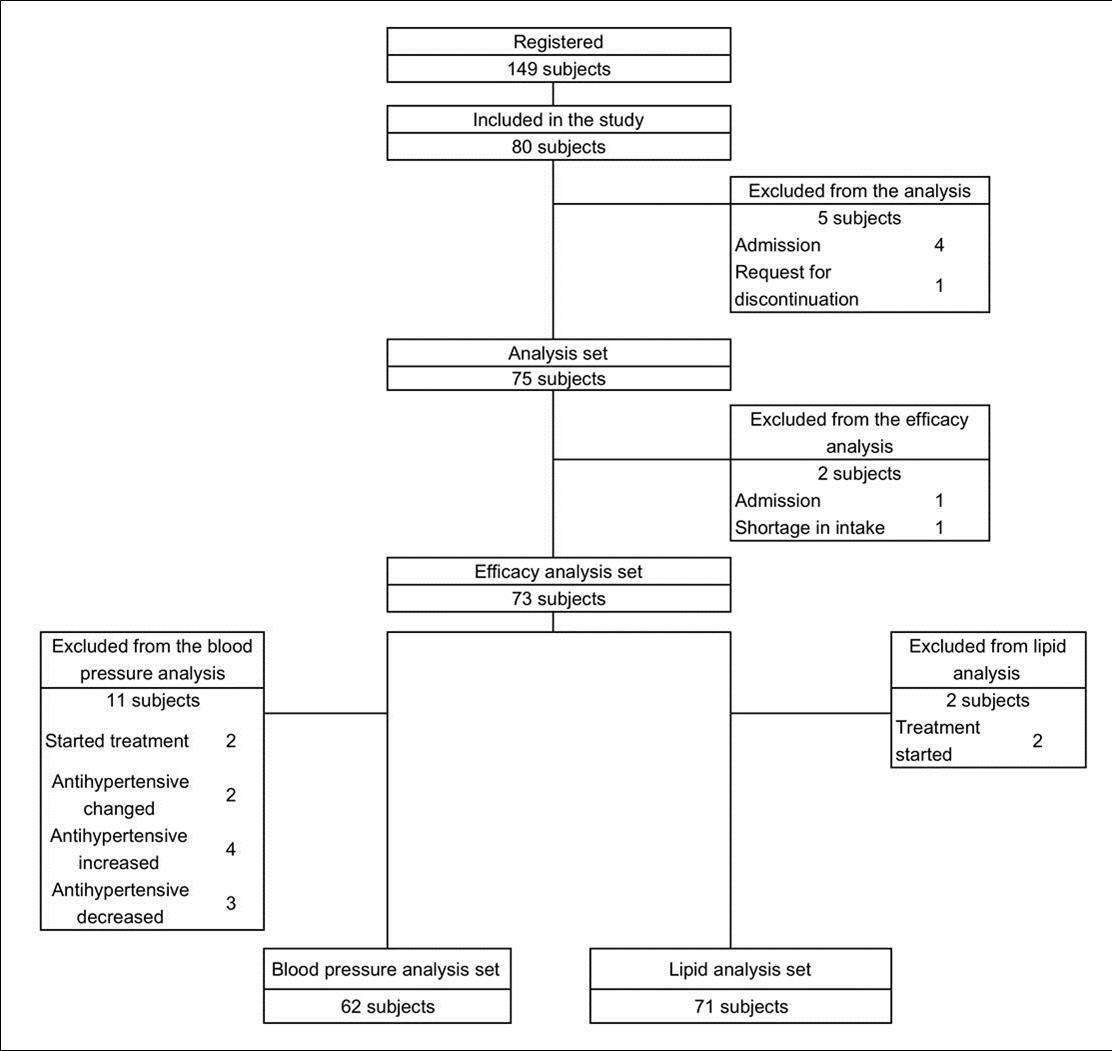
Characteristics of 80 subjects who met the criteria for this study based on the screening are shown in Table 2. 75 subjects, finally, were included in the analysis because 5 withdrew during the screening period (Table 2).
Table 2. Subject Characteristics at entry and baseline| Entry | Week 0 | ||
| No. of subjects | 80 | 75 | |
| Sex | Male (%) | 36 (45.0) | 34 (45.3) |
| Age (year) | Mean ± S.D. | 65.8 ± 6.9 | 65.4 ± 6.9 |
| Allergy | Yes (%) | 8 (10.0) | 8 (10.7) |
| Hypertension Treatment detail, n | Yes (%) | 40 (50.0) | 37 (49.3) |
| Yes (% of affected ) | 40 (100.0) | 37 (100.0) | |
| Exercise therapy | 5 | 5 | |
| Diet therapy | 8 | 8 | |
| Drug therapy | 36 | 33 | |
| Diabetes mellitus Treatment detail, n | Yes (%) | 8 (10.0) | 7 (9.3) |
| Yes (% of affected) | 7 (87.5) | 6 (85.7) | |
| Exercise therapy | 2 | 2 | |
| Diet therapy | 2 | 1 | |
| Drug therapy | 5 | 5 | |
| Dyslipidemia Treatment detail, n | Yes (%) | 29 (36.3) | 27 (36.0) |
| Yes (% of affected) | 22 (75.9) | 20 (74.1) | |
| Exercise therapy | 2 | 2 | |
| Diet therapy | 8 | 7 | |
| Drug therapy | 18 | 17 | |
| Physician's diagnosis/treatment* | Yes (%) | 56 (70.0) | 54 (72.0) |
| Currently taking medicine * | Yes (%) | 52 (65.0) | 50 (66.6) |
| Smoking | No (%) | 60 (75.0) | 56 (74.7) |
| Previous (%) | 13(16.3) | 12 (16.0) | |
| Current (%) | 7 (8.8) | 7 (9.3) | |
| Alcohol | No (%) | 39 (48.8) | 37 (49.3) |
| Sometimes (%) | 11 (13.8) | 11 (14.7) | |
| Frequently (%) | 30 (37.5) | 27 (36.0) | |
| BMI(kg/m2) | Mean ± S.D. | 23.3 ± 3.0 | 23.2 ± 3.0 |
| ≥25 (%) | 17 (21.3) | 15 (20.0) | |
| Abdominal circumference (cm) | Mean ± S.D. | 86.5 ± 8.0 | 85.6 ± 7.9 |
| ≥85 in males, | 31 (38.8) | 25 (33.3) | |
| ≥90 in females (%) | |||
| Laboratory blood pressure | |||
| SBP (mmHg) | Mean ± S.D. | 133.9 ± 12.9 | 138.7 ± 16.3 |
| ≥120 (%) | 69 (86.3) | 68 (90.7) | |
| DBP (mmHg) | Mean ± S.D. | 85.6 ± 8.9 | 88.3 ± 10.1 |
| ≥80 (%) | 60 (75.0) | 58 (77.3) | |
| Awakening home blood pressure | |||
| SBP (mmHg) | Mean ± S.D. | 135.4 ± 13.7 | |
| DBP (mmHg) | Mean ± S.D. | 84.0 ± 8.7 | |
| Bedtime home blood pressure | |||
| SBP (mmHg) | Mean ± S.D. | 128.7 ± 13.3 | |
| DBP (mmHg) | Mean ± S.D. | 78.1 ± 9.1 | |
| Pulse rate (beat/min) | Mean ± S.D. | 69.5 ± 9.5 | 66.7 ± 9.0 |
| Triglyceride (mg/dL) | Mean ± S.D. | 115.6 ± 67.0 | 117.5 ± 51.7 |
| ≥150 (%) | 17(21.5) | 14(18.7) | |
| HDL-C (mg/dL) | Mean ± S.D. | 60.2 ± 13.8 | 59.4 ± 13.7 |
| <40 (%) | 3(3.8) | 2(2.7) | |
| LDL-C (mg/dL) | Mean ± S.D. | 120.0 ± 25.9 | 115.0 ± 27.5 |
| ≥120 (%) | 40(50.0) | 36(48.0) | |
| FBS (mg/dL) | Mean ± S.D. | 94.4 ± 11.8 | 97.1 ± 12.9 |
| ≥110 (%) | 9(11.4) | 8(10.7) | |
| HbA1c (%) | Mean ± S.D. | 5.58 ± 0.44 | 5.63 ± 0.46 |
2. Efficacy Analysis
The summary statistics at entry, baseline (week 0), and end of intake (week 8) are shown in Table 2.
1) Effect on Laboratory-Based Examination of Blood Pressure
Time-course changes in ambulatory (laboratory) blood pressure are shown in Figure 2.
SBP at baseline (137.9 ± 16.4 mmHg) significantly increased from that at the time of entry (133.0 ± 13.1 mmHg). Further, SBP decreased to 129.2 ± 12.6 mmHg at the end of intake, which shows a significant decrease from baseline by −8.7 ± 12.1 mmHg (p < 0.0001). DBP at baseline (88.3 ± 10.3 mmHg) significantly increased from that of entry time (86.1 ± 8.4 mmHg). Subsequently, DBP decreased to 82.5 ± 8.2 mmHg at the end of intake, which shows a significant decrease from baseline by −5.8 ± 6.1 mmHg (p < 0.0001). The above findings demonstrated that consumption of kale powder significantly decreased laboratory-examined SBP and DBP.
Figure 2.Changes in Laboratory-based Examination of Blood Pressure. Laboratory-based examination of bllod pressure at entry, week 0, and week 8. * p<0.05 analyzed by paired Student’s t-test.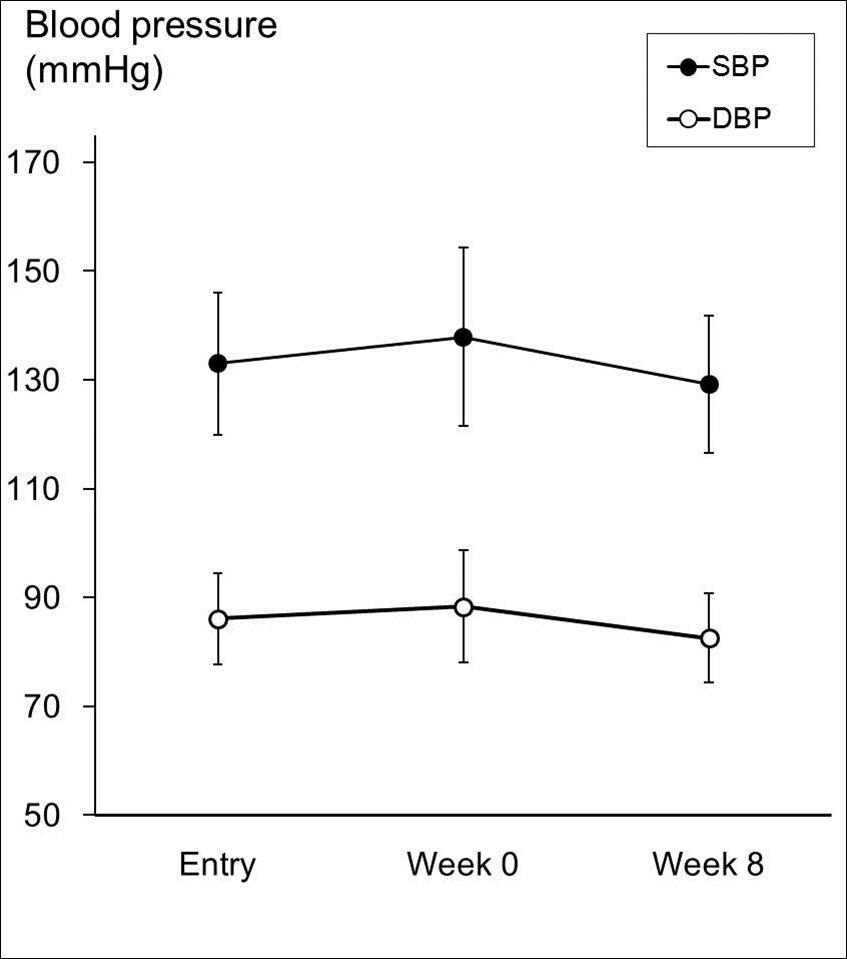
2) Time-Course Changes in Levels of The Blood Lipids (Triglycerides, LDL-C, and HDL-C)
Regarding triglycerides, no significant difference was observed between pre- and post-intake values. The difference between pre- and post-intake values was −5.4 ± 17.8 mg/dL for LDL-C and −2.5 ± 7.3 mg/dL for HDL-C, showing a significant decrease (p = 0.0130 and p = 0.0053, respectively). The above findings demonstrated that consumption of kale powder significantly decreased HDL-C and LDL-C levels.
3) Time-Course Changes in Fasting Blood Sugar and Hba1c Levels
Regarding fasting blood sugar levels, the difference between pre- and post-intake values was −1.8 ± 7.6 mg/dL, which shows a significant decrease (p = 0.0448). For HbA1c levels, no significant difference was observed between pre- and post-intake values. The above findings demonstrated that consumption of kale powder significantly decreased fasting blood sugar levels.
4) Time-Course Changes in BMI and Abdominal Circumference
Regarding BMI, no significant difference was observed between pre- and post-intake values. For abdominal circumference, the difference between pre- and post-intake values was −0.78 ± 2.44 cm, which shows a significant decrease (p=0.0081). The above findings demonstrated that consumption of kale powder significantly decreased abdominal circumference.
5) Effect on Home-Based Examination of Blood Pressure
Time-course changes in home-based examination of blood pressure are shown in Figure 3. Day-time SBP, measured at home by each subject in the morning before breakfast, started to decrease significantly from the week 2 of kale powder intake compared to baseline (p < 0.05). Further, day-time SBP at week 8 significantly decreased from baseline by −8.4 ± 7.7 mmHg (p < 0.0001). Day-time DBP started to decrease significantly from week 4 of kale powder intake compared to baseline (p < 0.05). Moreover, day-time DBP at week 8 significantly decreased from baseline by −4.4 ± 4.3 mmHg (p < 0.0001).
Figure 3.Time-course Changes in Home-based Examination of Blood Pressure. Each subject measured and recorded blood pressure after awakening (day-time) and before going to sleep (night-time) every day. *p<0.05 compared to baseline (week 0) using paired Student’s t-test.
Night-time SBP, measured before bed, started to decrease significantly from week 4 of kale powder intake compared to baseline (p < 0.05). Further, night-time SBP at week 8 significantly decreased from baseline by −6.0 ± 8.4 mmHg (p < 0.0001). Night-time DBP started to decrease significantly from week 6 of kale powder intake compared to baseline (p < 0.01). Moreover, bed-time DBP at week 8 significantly decreased from baseline by −3.2 ± 6.3 mmHg (p = 0.0002). The above findings indicated that it was beneficial to take kale powder for at least 4 weeks to achieve the desired effects.
3. Stratified Analysis of Laboratory-based Examination of Blood Pressure
For determination of the effect of kale powder on blood pressure, 62 subjects qualified for blood pressure analysis were further classified into the following three groups as shown in Figure 4. For each group, the stratified analysis was performed for the effects of kale powder consumption on laboratory-tested blood pressure.
Figure 4.Groups for Stratified Analysis of Blood Pressure. Grade I hypertension/normal blood pressure: SBP ≤159 and DBP ≤99, Grade II/III hypertension: SBP≤160 and/or DBP≤100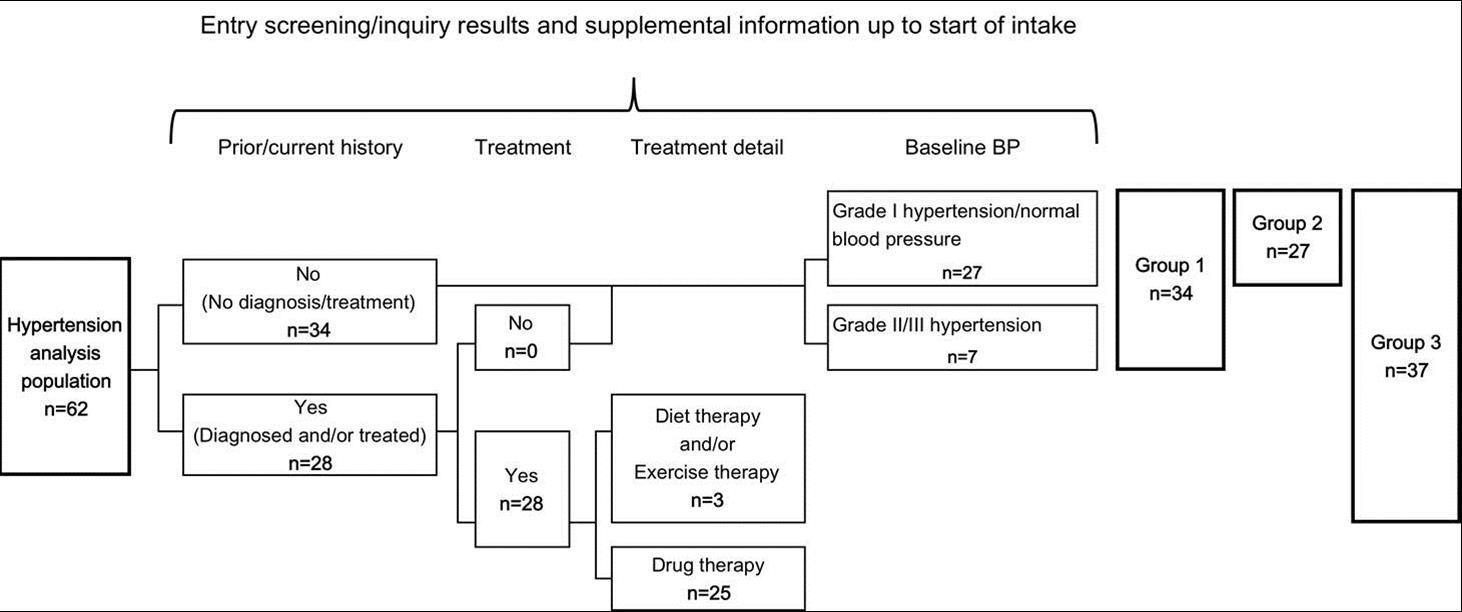
Group 1: subjects with no current treatment for hypertension (34 subjects)
Group 2: subjects with grade I hypertension/normal blood pressure (baseline SBP ≤ 159 mmHg and baseline DBP ≤ 99 mmHg) (27 subjects)
Group 3: subjects without drug therapy for hypertension (Group 2 + subjects without drug therapy for hypertension) (37 subjects)
Time-course changes in blood pressure at entry, baseline, and end of intake in the respective groups are presented in Figure 5.
Figure 5.Stratified Analysis of Time-course Changes in Laboratory-based Examination of Blood Pressure. Group 1: subjects with no current treatment for hypertension (n=34), Group 2: subjects with grade I hypertension/normal blood pressure (baseline SBP £ 159 mmHg and baseline DBP £ 99 mmHg) (n=27), Group 3: subjects without drug therapy for hypertension (Group 2 + subjects without drug therapy for hypertension) (n=37). *p<0.05 compared to week 0 in each group. (paired Student’s t-test)
In Group 1, SBP at the end of intake (125.5 ± 13.6 mmHg) significantly decreased (−9.6 ± 11.7 mmHg) from baseline, week 0 (135.1 ± 17.1 mmHg) (p < 0.0001). Additionally, DBP at the end of intake (82.4 ± 9.5 mmHg) significantly decreased (−6.2 ± 5.9 mmHg) from baseline (88.6 ± 11.5 mmHg) (p < 0.0001). In Group 2, SBP at the end of intake (122.1 ± 12.2 mmHg) significantly decreased (−6.6 ± 8.2 mmHg) from baseline (128.8 ± 12.8 mmHg) (p = 0.0003). Moreover, DBP at the end of intake (79.7 ± 8.1 mmHg) significantly decreased (−4.7 ± 5.4 mmHg) from baseline (84.4 ± 8.2 mmHg) (p = 0.0001). In Group 3, SBP at the end of intake (126.7 ± 13.9 mmHg) significantly decreased (−9.2 ± 11.3 mmHg) from baseline (135.9 ± 17.3 mmHg) (p < 0.0001). Additionally, DBP at the end of intake (82.6 ± 9.2 mmHg) significantly decreased (−6.2 ± 5.8 mmHg) from baseline (88.8 ± 11.3 mmHg) (p < 0.0001).
4. Safety
Although adverse events of kale powder, for which causality could not be excluded, included blood pressure increase and decrease in three subjects (4.0%) and hypertension and dyslipidemia in one subject each (1.3%), the incidence of all these adverse events was as low as < 5%. Regarding laboratory findings for which causality owing to the kale powder could not be excluded, the investigator determined that no such finding was observed on clinical use. Based on the laboratory tests (quantitative parameters/urinalysis), some parameters showed significant differences at the end of intake; however, no abnormal changes were reported. In conclusion, no safety concerns about kale powder were identified.
Discussion
Hypertension, hyperglycemia, and dyslipidemia are factors that can individually increase the risk for developing arteriosclerotic diseases. When these multiple factors are combined, the combination can synergistically increase the risk for developing arteriosclerotic diseases. Therefore, it has been considered that the recognition of the risk combination in earlier phase may contribute to control metabolic syndrome. Japan has a lower rate of obesity compared to other countries, though the rate of obesity has increased in Japan. The rate of obesity tends to decrease in young female individuals, but the rate in old individuals and young male individuals is increasing in the same manner (8). Although before the war, the primary Japanese diet comprised starch, after the war, the diet was westernized to excessive fats. In parallel with the change of diet, the rate of obesity has rapidly increased, which indicates the importance of diet therapy.
This prospective intervention study revealed the beneficial effect of the continuous intake of kale powder on metabolic syndrome in adult men and women with potential metabolic syndrome. A significant decrease was observed in laboratory and home test-based levels of blood pressure, levels of LDL-C, HDL-C, fasting blood sugar, and abdominal circumference. Particularly for blood pressure, home-based examination was conducted every week during the intervention period, and a significant decrease in all SBP and DBP values while awake and sleeping was observed, which corresponded to the result of laboratory-based blood pressure examination. Additionally, home test-based blood pressure decreased fourth week onwards, and a stable decrease was observed from fifth week onwards. These results clearly indicated that it is desirable to consume kale powder twice daily for at least 4 weeks to suppress increase in blood pressure.
As for the change of cholesterol, we found significant decrease of both LDL-C and HDL-C after 8 weeks of Kale powder intake. There are many reports that vegetables or this Kale decrease LDL-C, but we could not find any explanation for the mechanism of the decrease of HDL-C in this study. Previously, there is a report from Korea using Kale juice on sublinical hypertension patients, and found no decrease in HDL-C (7).
Furthermore, to assess the hypotensive effect of kale powder, the stratified analysis was performed based on laboratory-based examination of blood pressure. In groups 1 (subjects with no current treatment for hypertension), 2 (subjects with grade I hypertension/normal blood pressure), and 3 (subjects without drug therapy for hypertension), a significant decrease was observed in blood pressure at the end of diet intake compared to baseline, which demonstrates the antihypertensive effect of kale powder in subjects without blood pressure-related diseases.
While only a few studies have been published about the effect of kale on lifestyle-related diseases, kale powder has effects on blood cholesterol 5, blood sugar, and blood pressure 7. Therefore, the effect obtained in this study might be caused through the similar mechanisms of action; especially soluble fiber, potassium, and calcium may play important roles in the effects. Because polyphenols have been also reported to have the antihypertensive effect 8, 9, 10, it was suggested that polyphenols in kale might be associated with this effect. Furthermore, restriction of potassium leads to the increase of blood pressure probably due to sodium retention 11, and higher dietary potassium intake is considered to be associated with lowering blood pressure 12. Therefore, these results obtained in this study are not surprising, considering the facts that this kale powder contains 331 mg to 526 mg of potassium a day, which is comparable to 200 g of fresh shredded cabbage (more than a full of plate), and also contains 200 to 350 mg of calcium, which is equivalent to 300 g of milk. However, the precise mechanism of action should be further investigated.
Conclusion
In this study, the effect of 8-week continuous intake of kale powder on improvement of metabolic syndrome was investigated. The results showed the desirable effect of kale powder on laboratory and home test-based levels of blood pressure, fasting blood sugar, and abdominal circumference, which indicate its beneficial effect on metabolic syndrome. Additionally, the stratified analysis was performed for blood pressure evaluation, and a hypotensive effect was observed in all the groups of subjects without current treatment for hypertension, in those with grade I hypertension/normal blood pressure, and in those without drug therapy for hypertension. Furthermore, no safety concerns about kale powder were identified. In conclusion, it is clearly demonstrated that continuous intake of kale powder has desirable effects on metabolic syndrome without safety concerns.
References
- 1.Wakabayashi S. (1992) [The effects of indigestible dextrin on sugar tolerance: I. Studies on digestion-absorption and sugar tolerance]. , Nihon Naibunpi Gakkai Zasshi 68, 623-35.
- 2.Moreno Franco B, Leon Latre M, Andres Esteban EM, Ordovas J M, Casasnovas J A. (2014) Soluble and insoluble dietary fibre intake and risk factors for metabolic syndrome and cardiovascular disease in middle-aged adults: the AWHS cohort. , Nutr Hosp 30, 1279-88.
- 3.Hayakawa K, Kimura M, Kamata K. (2002) Mechanism underlying gamma-aminobutyric acid-induced antihypertensive effect in spontaneously hypertensive rats. , Eur J Pharmacol 438, 107-13.
- 4.Ma P, Li T, Ji F, Wang H, Pang J. (2015) Effect of GABA on blood pressure and blood dynamics of anesthetic rats. , Int J Clin Exp Med 8, 14296-302.
- 5.Kim S Y, Yoon S, Kwon S M, Park K S, Lee-Kim Y C. (2008) Kale juice improves coronary artery disease risk factors in hypercholesterolemic men. , Biomed Environ Sci 21, 91-7.
- 6.Tanaka Y, Okano J, Sekine K, Nomura R, Yuasa M. (2010) Fat intake and obesity among the Japanese. , J Oleo Science 10, 383-392.
- 7.Han J H, Lee H J, Kim T S, Kang M H. (2015) The effect of glutathione S-transferase M1 and T1 polymorphisms on blood pressure, blood glucose, and lipid profiles following the supplementation of kale (Brassica oleracea acephala) juice in South Korean subclinical hypertensive patients. , Nutr Res Pract 9, 49-56.
- 8.Chiva-Blanch G, Urpi-Sarda M, Ros E, Arranz S, Valderas-Martinez P. (2012) Dealcoholized red wine decreases systolic and diastolic blood pressure and increases plasma nitric oxide: short communication. Circ Res. 111, 1065-8.
- 9.Rattmann Y D, Anselm E, Kim J H, Dal-Ros S, Auger C. (2012) Natural product extract of Dicksonia sellowiana induces endothelium-dependent relaxations by a redox-sensitive Src- and Akt-dependent activation of eNOS in porcine coronary arteries. , J Vasc Res 49, 284-98.
- 10.Bnouham M, Benalla W, Bellahcen S, Hakkou Z, Ziyyat A. (2012) Antidiabetic and antihypertensive effect of a polyphenol-rich fraction of Thymelaea hirsuta L. in a model of neonatal streptozotocin-diabetic and N(G) -nitro-l-arginine methyl ester-hypertensive rats. , J Diabetes 4, 307-13.
Cited by (4)
This article has been cited by 4 scholarly works according to:
Citing Articles:
Frontiers in Nutrition (2024) OpenAlex
D. Aldisi, S. Sabico, Abeer A. Almiman, A. Al-farraj, T.A. Basaeed et al. - Frontiers in Nutrition (2024) Semantic Scholar
Frontiers in Nutrition (2024) Crossref
Plants (2021) OpenAlex
Erika Ortega-Hernández, M. Antunes-Ricardo, D. A. Jacobo-Velázquez - Plants (2021) Semantic Scholar
