Study of VCAM-1 Gene Expression in Normal and Tumoral Tissues in Patients with Colorectal Cancer
Abstract
Aim:
Colorectal cancer is one of the most commonly diagnosed cancers in the world. Cell adhesion molecules play an important role in the progression of various cancers. It has been shown that the high level expression of some Cell adhesion molecule could be a new diagnostic factor for several cancers.
Vascular cell adhesion molecule 1(VCAM1) is a cell surface glycoprotein that is expressed in the endothelium activated by cytokine. Generally, VCAM-1 expression level is very poor in normal adult tissue endothelial cells. According to the above explanation, this study was conducted to investigate the expression of VCAM-1 in tumoral tissues and adjacent normal tissues in Iranian colorectal cancer patients to its relation with clinicopathological Features in patients with cancer.
Methods:
In this study, 60 tumoral tissues and 39 adjacent normal tumor tissues were evaluated using reverse transcription-polymerase chain reaction (RT-PCR) technique.
Conclusion:
A significant correlation was found between VCAM-1 expression level and the stage, lymph nodes involvement, tumor progression factor of cancer and sex. Interestingly, VCAM-1 expression not observed in tumors with stage0. No association was seen between VCAM-1 expression and other clinical features such as age, size of the tumor, metastasis and the number of lymph nodes. These findings suggest that VCAM-1 expression level may reflected disease progression and elevation in VCAM-1 has prognostic significance in patients with colorectal carcinoma.
Author Contributions
Academic Editor: Ning Shen, Fulcrum Therapeutics
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Mahdiyeh Siyasi et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Colorectal cancer is the third most common cancer in the world with nearly 1.4 million new cases diagnosed in 20131.The same report shows that CRC in Iran between 2000 and 2009 has a five-year survival rate between 43 and 49 percent. CRC is normally a disease of aged people occurring in individuals over 65 years2. The relatively poor prognosis for colorectal cancer is due largely to the advanced stage of disease at the time of diagnosis. Therefore, early diagnosis is particularly important. Adhesion molecules (CAMs) and receptors from different tissues and organs vary and heterogeneity have been recognized in the mechanisms of tumor cell interaction with the endothelium.
One of the vital organs of the molecule, vascular cell adhesion molecule-1(VCAM-1) is highly conserved throughout evolution.VCAM-1 is an immunoglobulin (Ig)-like adhesion molecule with 7 extracellular Ig domains that are mainly expressed in endothelial cells 3,4. While expressed at a low level on resting endothelial cells, VCAM-1 is strongly induced by several inflammatory cytokines 5,6. Thus, VCAM-1 is a widely distributed protein. VCAM-1 acts in recognition of cell to cell, adhesion of endothelial cells-leukocytes, signal transmission, regulation of migration of leukocytes across the blood vessel wall and creating attachment points for the development of the endothelium during angiogenesis 7. It is possible that VCAM-1 is a candidate for mediating tumor cell adhesion to vascular endothelial cells and promoting the metastatic process. Recent reports have shown that angiogenesis favors tumor growth and facilitates entry of cells into the circulation8,9. VCAM-1 binds with high affinity to the integrin α4β1 (also known as very-late antigen, VLA-4) and α4β7.
Material and Method
Patients
Sixty specimens of colorectal cancer tissues were obtained from patient who underwent surgery at Hazrat Rasool Hospital and Imam Khomeini Hospital. Tumoral tissues and blood samples from patients with colorectal cancer (60) and Adjacent normal tissue as controls from colorectal cancer patients (39) were obtained after subjects provided informed consent 10. Tissue samples were frozen and stored at -70 degrees. The pathological information of all patients was obtained from Pathology Department of Hazrat Rasoll Hospital and Imam Khomeini Hospital. Staging of colorectal cancer was performed according to the International Union against Cancer (UICC) which is based on (AJCC-TNM) classification. They consisted of 29 males and 31 female patients ranging in age from 28 to 81 years. The project was approved by the local ethics committee of the National Institute for Genetic Engineering and Biotechnology (NIGEB).
RNA Extraction and cDNA Synthesis
RNA extraction was carried out with the Tripure Isolation Reagent (Roche Applied Sciences). For cDNA synthesis, 1 μg of total RNA from each sample was used to synthesize first-strand cDNA according to the manufacturer’s protocol (Ferments).
RNA Reverse Transcription-PCR
Evaluation of the expression level of VCAM-1 was performed by Reverse transcription-polymerase chain reaction (RT–PCR) with a one-step RT–PCR assay kit (Qiagen).The following primers were used for evaluating VCAM-1 expression: VCAM-1 forward 5’-AACCCAAACAAAGGCAGAG- 3’ VCAM-1 reversed 5’-CACAGGATTTTCGGAGCA- 3’.
GAPDH was selected as the housekeeping gene for assessment of expression. The primer sequences for GAPDH were as follows: forward 5’- GCAGGGGGGAGCCAAAAGGGT -3’ and reverse 5’- TGGGTGGCAGTGATGGCATGG -3’. Each 25-μl reaction mixture contained 5μl of cDNA solution in water, 0.6 μM of each primer (The concentration of primers used in the reaction was equivalent to 5 pM/μM , 6.3 mM water and 12.5 μL of Power SYBR Green PCR Master Mix. Cycle parameters were 94°C for 5 min to activate Taq, followed by 35 cycles of 94°C for 30 sec, 53 °C for 30 sec, and 72°C for 30sec. Finally, a cooling program cooled the reaction mixture to 40 °C. The products were underwent electrophoresis on 2% agarose gel.
Statistical Analysis
Statistical analysis was performed using the SPSS for software V20. The difference in Loss of gene expression VCAM-1 and gene expression VCAM-1 between Tumor and adjacent normal tissue samples in colorectal cancer patients were determined using the chi-square test. A P-value less than 0.05 was considered statistically significant.
Results
VCAM-1 Expression and Its Relation To Clinicopathologic Parameters
Colonic cancer tissue was obtained from 31 female and 29 male patients undergoing surgery for sporadic colon cancer. The Characteristic of patients are listed in Table 1.
TABLE 1. The characteristics of patients and relationships between expressions of vascular Cell molecule 1 and clinicopathologic factors in 60 colorectal patients| Variable | n | P vaiue(p<0.05) |
| Gender | ||
| Male | 29 | 0.032* |
| Female | 31 | |
| Age | ||
| ≥50 | 37 | 0.103 |
| <50 | 23 | |
| T classification | ||
| Tx | 4 | 0.000* |
| T1 | 0 | |
| T2 | 14 | |
| T3 | 34 | |
| T4 | 8 | |
| Tumor size | ||
| 1.5-3 | 16 | 0.0419 |
| 3.1-5 | 24 | |
| 5.6-12 | 20 | |
| Lymph node involvement | ||
| N0 | 27 | 0.073 |
| N1 | 24 | |
| N2 | 9 | |
| Lymph node metastasis | ||
| N0 | 25 | 0.014 |
| N1&N2 | 35 | |
| Distant metastasis | ||
| M0 | 47 | 0.276 |
| M1 | 13 | |
| UICC TNM classification | ||
| Stage0 | 3 | 0.000* |
| Stage1 | 17 | |
| Stage2 | 16 | |
| Stage3 | 17 | |
| Stage4 | 7 |
The average age of the patients was 50. Patients with average age older than 50 years were 61% (n=37) and average age of patients of younger than 50 years were 38% (n=23). There was no significant correlation between VCAM-1 expression level with age (P=0.103).
According to stages of the colorectal cancer that were histologically diagnosed, study of VCAM-1 expression in stage0 (n=3)5%, stage1 (n=17)28%, stage2 (n=16)26%, stage 3(n=17)28% and stage4 (n=7)12% of the patients was performed. In the present study data analysis showed that VCAM-1 expression level was correlated with stage of colorectal cancer (P=<0.000*).
In this study, there was no relation between VCAM-1 expression in colorectal cancer patients and tumor metastasis (than patients with primary tumors and non-metastatic colorectal cancer) (P=0.276).
The patients were divided into two groups according to tumor progression. The first group was based on the TNM classification. In This Category, VCAM-1 expression with tumor progression in the colon of walls was considerably significant (P=0.000*). Frequency classification of tumor progression included Tx (n=4, 7%), T2 (n=14, 23%), T3 (n=34, 57%) and T4 (n=8, 13%) in these patients, respectively. The second group was based on T1-T2 (n=20, 33%) and T3-T4 (n=40, 67%). The second group also had a significant association between expression VCAM-1 and tumor progression (P=0.003*).
Relationship between VCAM-1 expression and clinical risk factors of tumor size was measured in three groups. Most of the patients had tumor size between 1.5-3 cm (n=16) 27%, about 40% (n=24) of the patients had tumor size between3.1-5, While 33% (n=20) of the remaining patients between 5.6-12 cm. In the present study data analysis showed that VCAM-1 expression level was not correlated with tumor of size (p<0/419). Patients with lymph node involvement 58 % (N1&N2, n=35) and patients with non- lymph node involvement 42% (N0, n=25) were examined. Our results revealed that VCAM-1 expression level was correlated with lymph node involvement and non-lymph node involvement of the colorectal cancer (P=0.014*).
Expression of VCAM-1 in Normal Tissue and Tumoral Colon Cancer Tissue
In this study, VCAM-1 expression level in tumoral tissues (n=60) and adjacent normal tissue (n=39) (at least 5 cm away from the tumor margin) were examined. Expression of VCAM-1 was detected in all the tumoral tissues (100%) and half of the adjacent normal tissues of patients (33%).The expression level of VCAM-1 gene in colorectal cancer specimens and adjacent normal tissues were determined by RT-PCR assay and the final data were standardized against GAPDH mRNA levels in samples. The expression level of VCAM-1 in tumoral tissue was higher than normal (P=0.000*)
Significant correlation between VCAM-1 expression and age, tumor of size, number of lymph nodes involved and metastasis in patients had not been observed, as shown in Table 2.
TABLE 2. Correlation of VCAM-1 Gene Expression with cinicopathologic Characteristics of Patients| Expression of VCAM-1 | |
|---|---|
| Age | NS (P>0.05) |
| Gender | S(P<0.05) |
| Tumor progression | S(P<0.05) |
| Size of tumor | NS(P>0.05) |
| Lymph node involvement | S(P<0.05) |
| Number of lymph nodes | NS(P>0.05) |
| Stage | S (P<0.05) |
| Metastasis | NS(P>0.05) |
Discussion
Since cancer is a progressive and multifactorial disease, despite recent advances in prevention, the clinical outcome of diagnosis and treatment of the disease is far away from expectation yet. Due to the high incidence of colorectal cancer, early diagnosis is highly important 11.
Vascular cell adhesion molecule -1 (VCAM-1) had been noticed for the first time more than two decades ago as endothelial cell adhesion receptor, with key function for the recruitment of leukocytes in a cellular immune response. In the past few years, a growing insight into the molecular understanding of tumor genesity and metastasis of a variety of VCAM-1 functions in cancer have been elucidated. High expressions of vascular cell adhesion molecule-1 associated with several cancers have been reported. That can be used as a diagnostic factor and a new therapeutic target various cancer. According to the above description, this study was conducted to investigate relationship between VCAM-1 expression with clinicopathologic features in Iranian patients with colorectal cancer and the introduction of vascular cell adhesion molecule-1 as a potential therapeutic target and a biomarker of disease activity. VCAM-1 is a 90-kDa glycoprotein that contains six or seven immunoglobulin domains and belongs to the immunoglobulin superfamily of adhesion molecules. In principle, this gene as a molecule induction in human umbilical vein endothelial cells was detected which is capable of binding to tumor cell lines and lymphoid. VCAM-1 is constitutively expressed on many different types of endothelial and stromal cells and mediates In spread to lymph nodes is a common characteristic of carcinomas. It is also a critical factor to determine the next therapies for cancer. VCAM-1 expression was showed a significant correlation with lymph node involvement. But correlation between VCAM-1 expression with the number of lymph node involve in metastasis was not observed. Okugawa et al. measured tissue concentrations of the sVCAM-1 in tumor and normal mucosa which showed that there is a significant association between expression of vascular involvement and lymph duct 25 .One of metastasis to other organs in patients with colorectal cancer tumor growth in the intestinal wall and pass it around and invade nearby organs such as the liver. In this study, we found that Semi-quantitative VCAM-1 expression with tumor progression in the colon wall layers (with either grouping) a significant was observed. Relation of VCAM-1 expression with clinical risk factors of tumor size was examined. Statistically significant correlation between VCAM-1 expression and tumor size was not observed in these patients. However, in patients with tumor sizes between 5.6-12 cm VCAM-1 expressions was observed in all the patients. Here, can be stated that any size of colorectal tumors express VCAM-1 gene. But matter how the tumor size is greater VCAM-1 expression in these samples increases. The risk of colorectal cancer increases with advancing age. More than 90% of cases occur in people aged 50 or older. Significant correlation between VCAM-1 expression and the mean age of patients was not observed. Significant correlation between semi-quantitative expressions VCAM-1 with sex was observed in patients. We observed that in all patients female with VCAM-1 expression.
FIGURE 1.Comparison of vascular cell adhesion molecule 1 expression (VCAM-1) levels in 99 patients with colorectal cancer.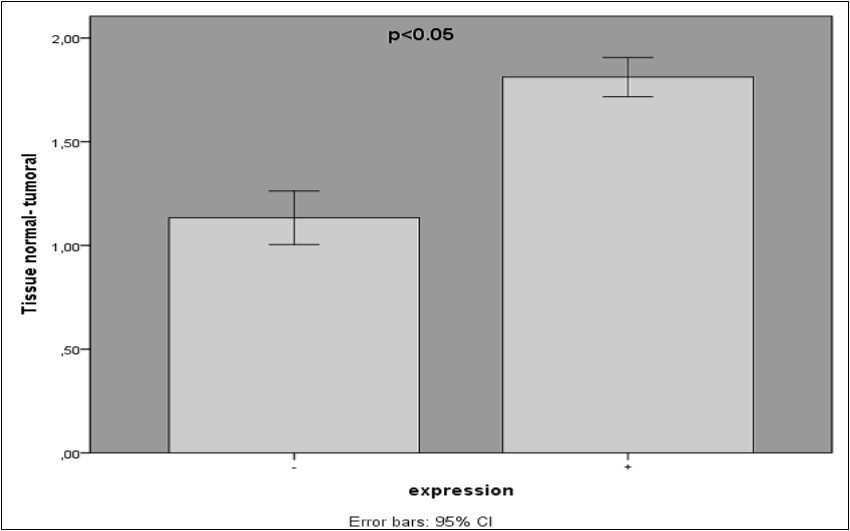
VCAM-1 may be involved in the progression of human colorectal carcinoma. Differentially expressed vascular molecules may influence the functional characteristics of prostate cancer has been reported 7, 15, 18, 19 .It is therefore conceivable that tumor cells that produce VCAM-1 might play a role in the adhesion of carcinoma cells to endothelium during disease progression. Alexiou et al have demonstrated that serum sVCAM-1 reflects tumor progression and metastasis in colorectal cancer 20. Kamezaki et al have reported that serum sVCAM-1 is extravagating leukocytes and represent new targets in colorectal cancer therapy. In general, an increasing number of studies on a variety of malignant diseases have suggested that VCAM-1 may play a role in the process of adhesion of tumor cells to endothelial cells and Neovascularization. Given that VCAM-1 in all tissues of the tumor and some normal samples expressed as a reliable diagnostic biomarker is not introduced. But if VCAM-1 expression was not observed in the specimens examined, it is likely that Normal or tumor tissue at stage0 is.
The controversial results which are obtained from several studies, show that serum levels of a variety of adhesion molecules, including VCAM-1 in cancer samples compared to normal samples has increased in various cancers 7, 15, 26, 27.
In conclusion, we report that expression of VCAM-1 is associated with lymph node involvement, lymph node metastasis, clinical stage, tumor progression in the colon wall layers and gender of patients in colorectal cancer. The correlation with clinicopathologic factors including tumor progression, and involvement and metastasis of lymph node, the crucial role of VCAM-1 in cancer cell adhesion and spreading, and blood vessel development and angiogenesis determines. Since only patients with zero or only one metastatic site were enrolled in this study, further investigation on patients with higher degree metastasis is recommended.
Acknowledgements
Research supported by NIGEB grants. The authors would like to thank all patients and healthy subjects who willingly participated in this study.
References
- 1.Haggar F A, Boushey R P. (2009) Colorectal cancer epidemiology: incidence, mortality, survival, and risk factors. Clinics in colon and rectal surgery. 22(4), 191.
- 2.Dolatkhah R, Somi M H, Bonyadi M J, Asvadi Kermani I, Farassati F et al.Colorectal Cancer in Iran: Molecular Epidemiology and Screening Strategies. , Journal of Cancer Epidemiology 2015-2015.
- 3.Vonderheide R H, Tedder T F, Springer T A, Staunton D E. (1994) Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. The Journal of cell biology. 125(1), 215-22.
- 4.Cybulsky M I, Fries J, Williams A J, Sultan P, Eddy R et al. (1991) Gene structure, chromosomal location, and basis for alternative mRNA splicing of the human VCAM1 gene. Proceedings of the National Academy of Sciences 88(17), 7859-63.
- 5.Luster A D, Alon R, von Andrian UH. (2005) Immune cell migration in inflammation: present and future therapeutic targets. Nature immunology. 6(12), 1182-90.
- 6.Osborn L, Hession C, Tizard R, Vassallo C, Luhowskyj S et al. (1989) Direct expression cloning of vascular cell adhesion molecule 1, a cytokine-induced endothelial protein that binds to lymphocytes. Cell. 59(6), 1203-11.
- 7.Maurer C A, Friess H, Kretschmann B, Wildi S, Müller C et al. (1998) Over‐expression of ICAM‐1, VCAM‐1 and ELAM‐1 might influence tumor progression in colorectal cancer. International journal of cancer. 79(1), 76-81.
- 8.Deng J, Liang H, Sun D, Zhang R, Zhan H et al. (2008) Prognosis of gastric cancer patients with node-negative metastasis following curative resection: outcomes of the survival and recurrence. , Canadian Journal of Gastroenterology 22(10), 835.
- 9.Lsegan M. (2005) New marker of angiogenesis CD105 (endoglin): diagnostic, prognostic and therapeutic role. Radiology and Oncology. 39-4.
- 10.Klöppel G, Rindi G, Perren A, Komminoth P, Klimstra D S. (2010) The ENETS and AJCC/UICC TNM classifications of the neuroendocrine tumors of the gastrointestinal tract and the pancreas: a statement. Virchows Archiv. 456(6), 595-7.
- 11.Pourhoseingholi M A. (2012) Increased burden of colorectal cancer in Asia. World journal of gastrointestinal oncology. 4-4.
- 12.Alexiou D, Karayiannakis A J, Syrigos K N, Zbar A, Sekara E et al. (2003) Clinical significance of serum levels of E-selectin, intercellular adhesion molecule-1, and vascular cell adhesion molecule-1 in gastric cancer patients. The American journal of gastroenterology. 98(2), 478-85.
- 13.Klemke M, Weschenfelder T, Konstandin M H, Samstag Y. (2007) High affinity interaction of integrin α4β1 (VLA‐4) and vascular cell adhesion molecule 1 (VCAM‐1) enhances migration of human melanoma cells across activated endothelial cell layers. Journal of cellular physiology. 212(2), 368-74.
- 14.Wu T. (2007) The role of vascular cell adhesion molecule-1 in tumor immune evasion. Cancer research. 67(13), 6003-6.
- 15.Okugawa Y, Miki C, Toiyama Y, Koike Y, Inoue Y et al. (2009) Serum level of soluble vascular cell adhesion molecule 1 is a valuable prognostic marker in colorectal carcinoma. Diseases of the Colon & Rectum. 52(7), 1330-6.
- 16.Byrne G J, Ghellal A, Iddon J, Blann A D, Venizelos V et al. (2000) Serum soluble vascular cell adhesion molecule-1: role as a surrogate marker of angiogenesis. , Journal of thenationalcancerinstitute 92(16), 1329-36.
- 17.Fukushi J-i, Ono M, Morikawa W, Iwamoto Y, Kuwano M. (2000) The activity of soluble VCAM-1 in angiogenesis stimulated by IL-4 and IL-13. The Journal of Immunology. 165(5), 2818-23.
- 18.Slack-Davis J K, Atkins K A, Harrer C, Hershey E D, Conaway M. (2009) Vascular cell adhesion molecule-1 is a regulator of ovarian cancer peritoneal metastasis. Cancer research. 69(4), 1469-76.
- 19.Christiansen I, Sundström C, Tötterman T H. (1998) Elevated serum levels of soluble vascular cell adhesion molecule‐1 (sVCAM‐1) closely reflect tumour burden in chronic B‐lymphocytic leukaemia. British journal of haematology. 103(4), 1129-37.
- 20.Alexiou D, Karayiannakis A, Syrigos K, Zbar A, Kremmyda A et al. (2001) Serum levels of E-selectin, ICAM-1 and VCAM-1 in colorectal cancer patients: correlations with clinicopathological features, patient survival and tumour surgery. , European Journal of Cancer 37(18), 2392-7.
- 21.Banner B F, Savas L, Woda B A. (1995) Expression of adhesion molecules in the host response to colon carcinoma. Ultrastructural pathology. 19(2), 113-8.
- 22.Velikova G, Banks R, Gearing A, Hemingway I, Forbes M et al. (1998) Serum concentrations of soluble adhesion molecules in patients with colorectal cancer. British journal of cancer. 77(11), 1857.
- 23.Lu X, Mu E, Wei Y, Riethdorf S, Yang Q et al. (2011) VCAM-1 promotes osteolytic expansion of indolent bone micrometastasis of breast cancer by engaging α4β1-positive osteoclast progenitors. Cancer cell. 20(6), 701-14.
- 24.Kemik O, Kemik A S, Hasirci I, Adas M, Purisa S et al.Serum level of soluble vascular adhesion molecule 1 in patients with rectal cancer. , Eur J Gen Med 8(2), 105-9.
- 25.Okugawa Y, Miki C, Toiyama Y, Koike Y, Yokoe T et al. (2010) Soluble VCAM-1 and its relation to disease progression in colorectal carcinoma. Experimental and Therapeutic Medicine. 1(3), 463-9.
Cited by (30)
This article has been cited by 30 scholarly works according to:
Citing Articles:
Cancer and Metastasis Reviews (2025) Crossref
Jess R Pickett, Yuao Wu, H. T. Ta - Cancer metastasis reviews (2025) Semantic Scholar
Cancer and Metastasis Reviews (2025) OpenAlex
Turkish Journal of Immunology (2024) Crossref
Turkish Journal of Immunology (2024) OpenAlex
Biochemical Pharmacology (2024) Crossref
Biochemical Pharmacology (2024) OpenAlex
International Journal of Molecular Sciences (2024) Crossref
Binura Perera, Yuao Wu, Jess R Pickett, Nadya Panagides, Francisca M. Barretto et al. - bioRxiv (2024) Semantic Scholar
International Journal of Molecular Sciences (2024) OpenAlex
Archives of Physiology and Biochemistry (2023) Crossref
Journal of Clinical Medicine (2022) Crossref
Kaijie Ren, Xin Xie, Tianhao Min, Tuanhe Sun, Haonan Wang et al. - Journal of Clinical Medicine (2022) Semantic Scholar
Journal of Clinical Medicine (2022) OpenAlex
Annals of Surgical Oncology (2022) Crossref
K. Mori, V. Schuettfort, S. Katayama, E. Laukhtina, B. Pradère et al. - Annals of Surgical Oncology (2022) Semantic Scholar
Annals of Surgical Oncology (2022) OpenAlex
Biomedicine & Pharmacotherapy (2022) Crossref
Harini Narasimhan, F. Ferraro, Andreas Bleilevens, R. Weiskirchen, E. Stickeler et al. - Biology (2022) Semantic Scholar
Biology (2022) Crossref
Biology (2022) OpenAlex
European Journal of Pharmacology (2021) Crossref
Ahmad Ghasemi, G. Vaseghi, Alaei Hojjatallah, S. Haghjooy Javanmard - Archives of Physiology and Biochemistry (2021) Semantic Scholar
Archives of Physiology and Biochemistry (2021) OpenAlex
Joanna Bronikowska, Małgorzata Kłósek, T. Janeczko, E. Kostrzewa-Susłow, Z. Czuba - Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie (2021) Semantic Scholar
Biomedicine & Pharmacotherapy (2021) OpenAlex
Pharmacological Research (2020) Crossref
Pharmacological Research (2020) OpenAlex
W. Xia, Imran Khan, Xiao-ang Li, Guoxin Huang, Zhiling Yu et al. - Pharmacological Research (2020) Semantic Scholar
Zhaoyu Fu, Jing Yu, Yan Liu, Bo Wu, Long Cheng et al. - (2020) Semantic Scholar
Research Square (Research Square) (2020) OpenAlex
Bioscience Reports (2020) Crossref
Xue Luo, Xiaolei Zhang, Jianming Peng, Yan Chen, Wenhui Zhao et al. - Bioscience Reports (2020) Semantic Scholar
Bioscience Reports (2020) OpenAlex
C. Erten, Aissa Houdjedj, H. Kazan - BMC Bioinformatics (2020) Semantic Scholar
bioRxiv (Cold Spring Harbor Laboratory) (2020) OpenAlex
European Journal of Pharmacology (2020) OpenAlex
Umer Ejaz, Fahad Akhtar, Jinbing Xue, Xinyu Wan, Tong Zhang et al. - European Journal of Pharmacology (2020) Semantic Scholar
International Journal of Molecular Sciences (2018) Crossref
Deok-Hoon Kong, Young Kwan Kim, M. R. Kim, J. Jang, Sukmook Lee - International Journal of Molecular Sciences (2018) Semantic Scholar
International Journal of Molecular Sciences (2018) OpenAlex