Effect of the Biofield Energy Treated Proprietary Test Formulation for Sleep Biomarkers in the Unpredictable Chronic Stress (UCS) Animal Model
Abstract
Sleep biomarkers in brain such as melatonin, BDNF (Brain-derived neurotrophic factor), PGD2 (Prostaglandin D2), leptin, orexin-A, and acetylcholine were evaluated in the unpredictable chronic stress (UCS) rodent model in the presence of Consciousness Energy Healing Treated (the Trivedi Effect®) novel test formulation in male Sprague Dawley (SD) rats using ELISA assay. The test formulation was consisted of minerals (Zn, Fe, Cu, Se, Ca, Mg), vitamins (C, E, B6, B12, D3), β-carotene, ginseng, and cannabidiol isolate. The test formulation constituents were divided into two parts, one part of each ingredient was distinct as the untreated test formulation, while the other portion of the test formulation and a group of animals received Biofield Energy Healing Treatment by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi. The level of melatonin in groups viz. G5 (Biofield Energy Treated Test formulation) and G7 (15-days pre-treatment of Biofield Energy Treated Test formulation) was significantly increased by 17.6% (p≤0.01) and 16%, respectively as compared with the disease control group (G2). Brain-derived neurotrophic factor (BDNF) level in brain was increased by 5.2% in G7 group as compared with the G4. Prostaglandins D2 (PGD2) level was significantly (p≤0.001) increased by 12.7%, 18.1%, 23.7%, and 30.7% in the G6, G7, G8 (15 days pre-treatment of Biofield Energy Treated Test formulation to the Biofield Energy Treatment per se rats), and G9 (untreated test formulation to the Biofield Energy Treatment per se to the rats) groups, respectively as compared with the G2. The level of leptin after Biofield Energy Treatment and with the test formulation was altered. However, orexin-A level was significantly decreased by 37.1% (p≤0.05), 32.6%, 40.5% (p≤0.05), 44.4% (p≤0.05), and 28.2% in the G5, G6, G7, G8, and G9 groups respectively, as compared with the G2. Similarly, acetylcholine (Ach) level was significantly (p≤0.001) decreased by 42.5%, 49.2%, 40.1%, 47.9%, and 45% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2. Overall, the results showed the significant slowdown the stress-related disease progression and its complications/symptoms in the preventive in the Biofield Energy Treatment group per se and/or Biofield Energy Treated Test formulation groups (viz. G6, G7, G8, and G9) comparatively with the disease group. The Trivedi Effect® showed increased level of melatonin and decreased levels of insomnia related brain biomarkers which might be helpful to induce better sleep in human.
Author Contributions
Academic Editor: Qiang Cheng, Biomedical Informatics Institute, and Computer Science Department University of Kentucky Lexington, China.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Mahendra Kumar Trivedi, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Stress could be considered as a nonspecific and adaptive response that may occur due to some social environmental factors. It may induce some kind of mental disorders, in which the patients exhibited cognitive problems and persistent fatigue such as lack of concentration and memory loss. According to the Swedish National Board of Health and Welfare, this fatigue condition could be described as Exhaustion Disorder (ED) that may apparently caused by prolonged stress without adequate recovery 1. According to the diagnostic criteria, ED may occur after the long-term stress i.e., at least six months duration of persistent stress. The symptoms of stress could be characterised as, sleep disturbance, mental and physical fatigue, irritability, cognitive problems, depressed mood, and reduced stress tolerance 2. The human body has the unique property that helps in the efficient tolerance of stress; however, it requires the rapid activation of the HPA axis, as well as the effective termination of that activation in absence of stressor or if it remains present for a long time 3, 4. Moreover, the commencement of both important processes largely depends on the modulation of the brain by the nervous, immune, and endocrine systems 5, 6. Besides, stress may cause severe harm to brain due to its high sensitivity; and it may cause some mental and somatic diseases, in case the stress intensity exceeds the tolerable limits 7, 8. Various scientific studies reported that chronic stress could act as a risk factor for the development of cognitive deficits, such as Alzheimer’s disease (AD). The research studies showed that chronic stress may impair the early long-term potentiation (LTP) in the at-risk rat model 9, and may also increase amyloid-β plaque deposition as well as decrease the hippocampal synaptic plasticity in Tg2576 mice 10, 11. Besides, some researchers also confirmed that chronic stress exposure may increase the glucocorticoids levels, neuronal injury, promoted senile plaque deposition, and cognitive impairment in mice models 12.
In this regard, melatonin acts in regulation of many circadian aspects such as, the sleep-wake cycle and also protects the cells against oxidative stress. It is known to possess the characteristics of a free radical scavenger, and helps in reducing the oxidative stress 13. The other functions of melatonin in body involve protection of neurons against oxidative stress, depression, ischemia, and cognitive impairment. It was observed that the stress may affect the melatonin level in body as the scientists characterized the link between melatonin and AD; and they reported its correlation with cognitive impairment in the aging population due to the low melatonin levels at night 14, 15. Another important agent in body is orexin that mainly functions in promoting and stabilizing the wakefulness 16, 17. The orexinergic neurons are present in the hypothalamus, which project their fibres to various other nuclei such as the septal nuclei, LC, medullary reticular formation, etc., that govern the sleep-wake cycle 18. In addition, orexin may also act in modulating the circadian oscillation of AD risk genes such as ABCA1, APOE, GSKβ, BACE1, etc., 19, which establishes its role in stress induced mental impairments. There are various pharmaceutical and nutraceutical formulations that have been developed by the scientific community to solve the complications arising due to Unpredictable Chronic Stress (UCS), either by rectifying the root cause or improving the associated symptoms 20.
In this research project, the novel test formulation was studied for its effect on the UCS- induced stress and sleep disorders in Sprague Dawley rats. Furthermore, the test formulation was treated with the Biofield Energy Treatment, which is considered as a Complementary and Alternative Medicine (CAM), by a renowned Biofield Energy Healer. Biofield Energy Healing is a novel approach that has been known widely due to its significant impact against various pathological conditions 21; and therfore, accepted worldwide. CAM therapies are considered as the one among the best alternative complementary health treatment approach by the National Center for Complementary/Alternative Medicine (NCCAM) 22, due to its several benefits over the current preferred treatment approach 23. Other than the Biofield Energy Healing, there are various other CAM therapies, medicines and practices such as Tai Chi, deep breathing, yoga, natural products, therapeutic touch, Qi Gong, Reiki, pranic healing, polarity therapy, chiropractic manipulation, special diets, meditation, homeopathy, mindfulness, progressive relaxation, movement therapy, pilates, Ayurvedic medicine, and traditional Chinese herbs and medicines in biological systems, etc 24, 25. In the similar way, the Trivedi Effect®-Consciousness Energy Healing therapy has been known worldwide as a Conventional therapy due to its significant impact in various living and non-living objects. The impact of the Trivedi Effect® has been scientifically studied in various scientific fields such as, agriculture science 26, materials science 27, 28, microbiology 29, 30, biotechnology 31, 32, skin health 33, 34, bioavailability studies 35, 36, dietary supplement 37, bone health 38, 39, 40, cancer research 41, and overall human health and wellness. In this study, the authors sought to study the impact of the Biofield Energy Treatment (the Trivedi Effect®) for the level of brain sleep biomarker such as melatonin, BDNF (Brain-derived neurotrophic factor), PGD2, Leptin, Orexin-A, and acetylcholine in Sprague Dawley rats using ELISA assays.
Material and Methods
Chemicals and Reagents
The novel test formulation designed was constituted with pyridoxine hydrochloride (vitamin B6), calcitriol, zinc chloride, magnesium (II) gluconate, and β-carotene (retinol, Provit A), which were purchased from TCI, Japan. Copper chloride, cyanocobalamin (vitamin B12), calcium chloride, vitamin E (alpha-tocopherol), cholecalciferol (vitamin D3), iron (II) sulfate, and sodium carboxymethyl cellulose (Na-CMC) were procured from Sigma-Aldrich, USA. Ascorbic acid (vitamin C) and sodium selenate were obtained from Alfa Aesar, India. Cannabidiol isolate and Panax ginseng extract were obtained from Panacea Phytoextracts, India and Standard Hemp Company, USA, respectively. Imipramine Hydrochloride was purchased from Sigma, USA. For the estimation of brain sleep biomarker such as melatonin, BDNF (Brain-derived neurotrophic factor), PGD2, Leptin, Orexin-A, and acetylcholine, specific ELISA kits were used such as for detection, which were procured from CUSABIO and My Bio Source, USA respectively.
Maintenance of Animal
Randomly breed male Sprague Dawley (SD) rats with body weight ranges from 200 to 300 gm were used in this study. The animals were purchased from M/s. Vivo Bio Tech, Hyderabad, India. Animals were randomly divided into nine groups based on their body weights consist of 6 animals of each group. They were kept individually in sterilized polypropylene cages with stainless steel top grill having provision for holding pellet feed and drinking water bottle fitted with stainless steel sipper tube. The animals were maintained as per standard protocol throughout the experiment.
Consciousness Energy Healing Strategies
The test formulation consisted each constituents was divided into two parts. One part of each ingredients of the test formulation did not receive any sort of treatment and was labeled as the untreated or control sample, while the second part of each ingredients of the test formulation was treated with the Trivedi Effect® - Energy of Consciousness Healing Treatment (Biofield Energy Treatment) by a renowned Biofield Energy Healer, Mr. Mahendra Kumar Trivedi under standard laboratory conditions for ~3 minutes. The novel test formulation was consisted of zinc chloride, iron (II) sulfate, copper chloride, vitamin B6, vitamin B12, vitamin D3, sodium selenate, calcium chloride, ascorbic acid, vitamin E, beta carotene, Panax ginseng extract, cannabidiol, and magnesium (II) gluconate. Besides, three group of animals also received Biofield Energy Healing Treatment (known as the Trivedi Effect®) by Mr. Mahendra Kumar Trivedi under similar laboratory conditions for ~3 minutes in the research laboratory of Dabur Research Foundation, New Delhi, India. The energy transmission was done without touching the samples or animals. After that, the Biofield Energy Treated samples was kept in the similar sealed condition and used as per the study plan. In the same manner, the control test formulation group was subjected to “sham” healer under the same laboratory conditions for comparison purposes. The “sham” healer did not have any knowledge about the Biofield Energy Treatment. The Biofield Energy Treated animals were also taken back to experimental room for further proceedings.
Experimental Test Procedure
Seven days after acclimatization, animals were randomized and grouped based on the body weight. The test formulation was prepared freshly prior to dosing and administered to the animals using an oral intubation needle attached to an appropriately graduated disposable syringe. The dose volume was 10 mL/kg in morning and evening based on body weight. The experimental groups were divided as G1 as normal control; G2 as disease control (UCS: Unpredictable Chronic Stress with 0.5% CMC); G3 as reference item (UCS along with imipramine hydrochloride, 30 mg/kg); G4 includes UCS along with untreated test formulation; G5 as UCS along with Biofield Energy Treated test formulation); G6 group includes UCS along with Biofield Energy Treatment per se to animals from day -15; G7 as UCS along with Biofield Energy Treated test formulation from day -15; G8 group includes UCS along with Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9 group denoted UCS along with Biofield Energy Treatment per se animals plus untreated test formulation. G1 and G2 animals were treated orally with 0.5% w/v CMC-Na in distilled water for 8 weeks (From day 1 to 56). Group G3 animal was treated orally with reference item, imipramine hydrochloride at a dose of 30 mg/kg body weight for 8 weeks. The freshly prepared suspensions of untreated test formulation and Biofield Energy Treated Proprietary Product was administered orally to the G4 and G5 group animals, respectively, at a dose of 1257.80 mg/kg body weight in the morning and 2012.75 mg/kg body weight in the evening, respectively for 8 weeks. G6 group was not to be dosed with the test formulation. In addition; G7 and G8 group were dosed similar to the G4 and G5 dosing regimen, but from the day of Biofield Energy Treatment (i.e. from day-15 to day 56). G9 group, Biofield Energy Treated per se animal was treated with untreated test formulation for 8 weeks. Body weight and clinical signs were taken daily throughout the experimental period. All the animals except G1 group received stress induced procedures such as stress procedures like sound stress, tilted cages and crowd stress, cold and warm water swim stress, food and water deprivation, stress due to change in the light and dark cycle were undergo seven different types of unpredictable stress procedures after scheduled dosing daily at specified interval to the end of the experiment for 8 weeks after the initiation of stress, which vary every week interval i.e. shuffling of stress type. At the end of (8 week) experimental period, all the animals were individually subjected gross necropsy to collect brain tissue for the experimental purpose.
Estimation of Brain Sleep Biomarker (Melatonin, BDNF, PGD2, Leptin, Orexin-A, and Acetylcholine)
The brain homogenate from all the animals groups after experimental period was subjected for the estimation of sleep biomarker such as melatonin, BDNF, PGD2, Leptin, Orexin-A, and acetylcholine was performed. The entire assay was estimation using ELISA method as per manufacturer’s recommended standard procedure. This was a quantitative method and the principle was based on the binding of protein and their specific antibody.
Statistical Analysis
The data were represented as mean ± standard error of mean (SEM) and subjected to statistical analysis using Sigma-Plot statistical software (Version 11.0). For multiple comparison One-way analysis of variance (ANOVA) followed by post-hoc analysis by Dunnett’s test and for between two groups comparison Student’s t-test was performed. The p≤0.05 was considered as statistically significant.
Results and Discussion
Estimation of Brain Melatonin
Melatonin, a ubiquitous natural neurotransmitter secreted in the brain by the pineal gland with most diverse biological actions such as energy metabolism, circadian rhythm, and the immune system 42. It imparts significant protective action on neurons, especially against wide variety of oxidative stress by removing reactive oxygen, which suggest its role against many targets for the development of many neurological diseases 43, 44. Melatonin in CNS also reacts with various reactive intermediates in order to protect against tissue damage 45. Brain melatonin level was estimated in all the experimental test groups and the results are presented in Figure 1. The data showed the melatonin level in the unpredictable chronic stress (G2) group was 75.31 ± 4.1 pg/mL, which was significantly decreased by 30.9% as compared with the normal control (G1, 109.04 ± 3.4 pg/mL). Imipramine treatment (G3) increased brain melatonin level (80.37 ± 4.4 pg/mL) by 6.7% as compared to the G2. The untreated test formulation to the untreated rats (G4) showed an increased brain melatonin level (78.98 ± 0.5 pg/mL) by 4.9% as compared to the G2. The Biofield Energy Treated test formulation to the untreated rats (G5) showed a significant increased brain melatonin level (88.58 ± 4.2 pg/mL) by 17.6% (p≤0.01) and 12.2% as compared to the G2 and G4 groups, respectively. Biofield Energy Treatment per se to the rats (G6) increased the brain melatonin level (84.45 ± 2.0 pg/mL) by 12.1% and 6.9% as compared to the G2 and G4 groups, respectively. 15-days pre-treatment of Biofield Energy Treated test formulation (G7) increased the brain melatonin level (87.39 ± 4.1 pg/mL) by 16.0% and 10.7% as compared to the G2 and G4 groups, respectively. 15-days pre-treatment of Biofield Energy Treated test formulation to the Biofield Energy Treated rats (G8) showed decreased brain melatonin level (72.82 ± 0.8 pg/mL) as compared to the G2 and G4. The untreated test formulation to the Biofield Energy Treated rats (G9) decreased brain melatonin level (73.85 ± 2.3 pg/mL) as compared to the G2 and G4.
Figure 1.The effect of the test formulation on the level of brain melatonin in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30 mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). **p≤0.01 vs. G2.
Estimation of Brain-derived neurotrophic factor (BDNF)
BDNF, a neurotrophin which is important for growth, survival, and neuron maintenance as an important mechanism involved in emotional and cognitive function. BDNF was found to be very protective to neuron in stress situations, which is the leading cause of death globally with total case of more than 300 millions. BDNF role has been widely correlated in depression, aging, and posttraumatic stress disorder 46, 47. Brain BDNF level was measured in all the experimental groups and is graphically presented in the Figure 2. Brain BDNF level (7.15 ± 0.30 pg/mL) in the G2 was significantly decreased by 8.3% as compared to the normal control (G1, 7.79 ± 0.27 pg/mL). Imipramine (G3) showed significantly increased brain BDNF level (7.52 ± 0.20 pg/mL) by 5.2% as compared to the G2. G4 (7.02 ± 0.56 pg/mL) and G6 (6.75 ± 0.40 pg/mL) groups showed a decreased brain BDNF level as compared to the G2. G7 (7.38 ± 0.38 pg/mL) group showed an increased brain BDNF level by 3.3% and 5.2% as compared to the G2 and G4 groups, respectively. G8 (5.64 ± 0.38 pg/mL) and G9 (6.28 ± 0.31 pg/mL) groups showed a decreased brain BDNF level as compared to the G2.
Figure 2.The effect of the test formulation on the level of brain BDNF in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30 mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).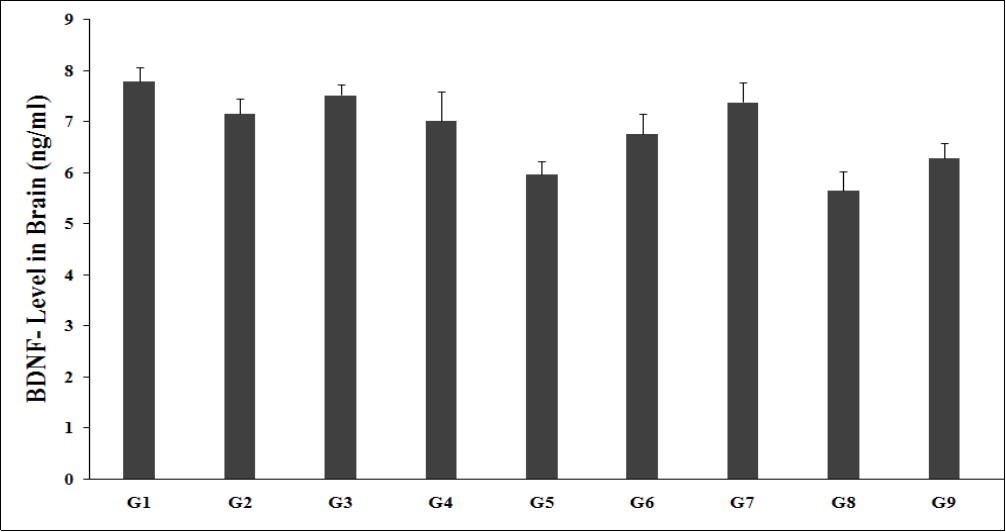
Estimation of Brain PGD2
Prostaglandin D2 (PGD2), a prostaglandin (PG), which is most abundant PGs involved in various physiological processes in the mammalian brain. It is mostly produced in organs, such as the brain, bone marrow, thymus, spleen, oviduct, uterus, prostate, ovary, testis, and epididymis. PGD2 is involved in major depressive disorder as one of the main physiological and pharmacological role in the central nervous system. Various reports suggest that the level of PGD2 was decreased in stressed conditions and depressive disorder physiology. Thus, the level of PGD2 can be maintained by using some alternative way of treatment would be the best mode of treatment in depressive disorders 48. All the test groups were tested for the presence of brain PGD2 level, which was measured and was graphically presented in the Figure 3. Brain PGD2 level (0.80 ± 0.01 ng/mL) in the G2 was significantly decreased by 11.6% as compared to the normal control (G1, 0.91 ± 0.02 ng/mL). Imipramine (G3) showed significantly increased brain PGD2 level (0.85 ± 0.02 ng/mL) by 6.3% as compared to the G2. G4 (0.85 ± 0.01 ng/mL) group showed an increased brain PGD2 level by 6.2% as compared to the G2. G6 (0.90 ± 0.01 ng/mL), G7 (0.95 ± 0.02 ng/mL), G8 (0.99 ± 0.03 ng/mL), and G9 (1.05 ± 0.01 ng/mL) groups showed a significant increased brain PGD2 level by 6.1%, 11.2%, 16.6%, and 23.1%, respectively, as compared with the G4, untreated test formulation group. Prostaglandins D2 (PGD2) level was significantly (p≤0.001) increased by 18.1%, 23.7%, and 30.7% in the G7, G8 (15 days pre-treatment of Biofield Energy Treated Test formulation to the Biofield Energy Treatment per se rats), and G9 (untreated test formulation to the Biofield Energy Treatment per se to the rats) groups, respectively as compared with the G2.
Figure 3.The effect of the test formulation on the level of brain PGD2 in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30 mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). ***p≤0.001 vs. G2.
Estimation of Brain Leptin
Leptin, is a hormone mainly released in adipose tissue from fat cells, it provide signal to the brain especially in the hypothalamus region. Its role in weight loss and its relation with appetite and high intake of food has been widely studies 49. However, it has been found to have significant role in extra-hypothalamic effects, which protects the brain/CNS from various form of neurodegenerative disorders like Alzheimer’s disease or the development of the mood disorders. Thus, regulation of leptin level in CNS is the core area of research now a days for various neurodegenerative disorders due to the action of leptin on neuron structure and function, as well as on glial cells 50, 51, 52. The level of brain leptin level was estimated among all the experimental groups and is graphically presented in the Figure 4. Brain leptin level (7.24 ± 0.30 ng/mL) in G2 group was significantly increased by 42.3% as compared to the normal control (G1, 5.09 ± 0.36 ng/mL). Imipramine (G3) showed significantly decreased the brain leptin level (6.96 ± 0.44 ng/mL) by 3.9% as compared to the G2. G4 (6.18 ± 0.28 ng/mL) group showed an increased brain leptin level by 6.2% as compared to the G2. G5 (5.01 ± 0.42 ng/mL), G6 (4.31 ± 0.24 ng/mL), G7 (4.69 ± 0.31 ng/mL), and G8 (4.83 ± 0.39 ng/mL) groups showed a significant decreased brain leptin level by 18.9%, 30.2%, 24%, and 21.8% respectively, as compared with the G4, untreated test formulation group. However, brain leptin was also significantly decreased by 30.8%, 40.5%, 35.2%, 33.3%, and 15.6% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2 group.
Figure 4.The effect of the test formulation on the level of brain leptin in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30 mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6).
Estimation of Brain Orexin-A
Orexin (also known as hypocretin) is a kind of peptide released in neurons of the peripherical, lateral, and posterior hypothalamus, it regulates sleep/wakefulness cycle and feeding behaviour. Besides, brain leptin also helps in coordination of emotion, energy homeostasis, reward system, and arousal. Thus, it has been found to have its important in various CNS disorder due to its huge biological role in sleep cycle or awake 53, 54. The level of brain orexin-A level was estimated among all the experimental groups and is graphically presented in the Figure 5. Brain orexin-A level (205.76 ± 22.3 ng/mL) in G2 was significantly increased by 71.9% as compared to the normal control (G1, 119.73 ± 11.6 ng/mL). Imipramine (G3) showed significantly decreased brain orexin-A level (169.48 ± 19.7 ng/mL) by 17.6% as compared to the G2. G4 (162.96 ± 24.4 ng/mL) group showed an increased brain orexin-A level by 20.8% as compared to the G2. G5 (129.34 ± 21.7 ng/mL), G6 (138.59 ± 8.5 ng/mL), G7 (122.5 ± 23.4 ng/mL), G8 (122.50 ± 23.4 ng/mL), and G9 (147.66 ± 19.6 ng/mL) groups showed a significant decreased brain orexin-A level by 20.6%, 15%, 24%, 29.8%, and 9.4%, respectively as compared with the G4, untreated test formulation group. However, brain orexin-A was also significantly decreased by 37.1% (p≤0.05), 32.6%, 40.5% (p≤0.05), 44.4% (p≤0.05), and 28.2% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2 group.
Figure 5.The effect of the test formulation on the level of brain orexin-A in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30mg/kg); G4: (UCS + untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). *p≤0.05 vs. G2.
Estimation of Brain Acetylcholine (Ach)
The neurotransmitter acetylcholine, is a chemical messenger that actions on skeletal mUCSles to contract. It is released by nerve cells in the brain, and found in between the nerve synapses, or gaps, between nerve cells. It plays an important role in signalling for mUCSle movement, learning and memory formation, sensation of pain, endocrine system regulation, and rapid eye movement (REM) sleep cycles. Degeneration of the acetylcholine pathway associated with the pathologies of Alzheimer's disease 55. The level of brain Ach level was estimated among all the experimental groups and is graphically presented in the Figure 6. Brain Ach level (456.29 ± 11.1 pg/mL) in G2 was significantly increased by 29.4% as compared to the normal control (G1, 352.70 ± 11.1 pg/mL). Imipramine (G3) showed significantly decreased brain Ach level (424.28 ± 32.8 pg/mL) by 7% as compared to the G2. G4 (441.86 ± 15.8 pg/mL) group showed a decreased brain Ach level by 3.2% as compared to the G2. G5 (262.53 ± 11.2 pg/mL), G6 (231.80 ± 7.5 pg/mL), G7 (273.34 ± 23.2 pg/mL), G8 (237.80 ± 10.5 pg/mL), and G9 (251.06 ± 12.4 pg/mL) groups showed a significant decreased brain Ach level by 40.6%, 47.5%, 38.1%, 46.2%, and 43.2%, respectively as compared with the G4, untreated test formulation group. However, brain Ach was also significantly (p≤0.001) decreased by 42.5%, 49.2%, 40.1%, 47.9%, and 45% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2 group.
Figure 6.The effect of the test formulation on the level of brain Ach in Sprague Dawley rats. G: Group; G1: Normal control; G2: Disease control (UCS: Unpredictable Chronic Stress + 0.5% CMC); G3: Reference item (UCS + Imipramine hydrochloride 30 mg/kg); G4: (UCS + Untreated test formulation); G5: (UCS + Biofield Energy Treated test formulation); G6: (UCS + Biofield Energy Treatment per se to animals from day -15; G7: (UCS + Biofield Energy Treated test formulation from day -15); G8: (UCS + Biofield Energy Treatment per se plus Biofield Energy Treated test formulation from day -15), and G9: (UCS + Biofield Energy Treatment per se animals plus untreated test formulation). Values are presented as mean ± SEM (n=6). ***p≤0.001 vs. G2.
In this research plan, four groups were considered as preventive maintenance groups. These groups were G6 (Biofield Energy Treatment per se to animals at -15 days), G7 (Biofield Energy Treated test formulation from day -15), G8 (Biofield Energy Treatment per se to animals along with Biofield Treated test formulation from day -15), and G9 (Biofield Treatment per se at -15 days to animals with untreated test formulation). The results showed the significant slowdown of the disease progression, stress disease related all other symptoms/complications and also reduced the chances of disease sUCSeptibility in these groups. Specifically, group G6 (preventive Biofield Energy Treatment group per se at -15 days) showed the best results as a prophylactic/preventive treatment group compared to the other groups. Based on the overall data, it suggests that the Biofield Energy Healing Therapy was found to be most effective and benefited in order to prevent and protect from the occurrence of any type of diseases in rat model. It indicated that this therapy can act as a preventive maintenance therapy to prevent the occurrence of the disease, slow down the disease progression and disease related complications of the existing aliments that will ultimately improve the overall health and quality of life in human.
Conclusions
Sleep biomarker in brain was identified and major biomarkers were estimated after treatment with the Biofield Energy Treated test formulation and Biofield Energy per se on the animal. The following parameters such as the level of melatonin, BDNF (Brain-derived neurotrophic factor), PGD2 (Prostaglandin D2), leptin, orexin-A, and acetylcholine in the unpredictable chronic stress (UCS) rodent model were estimated. Melatonin level in brain was measured and reported to be significantly increased by 17.6% (p≤0.01) and 16% in the G5 (Biofield Energy Treated Test formulation to the untreated rats) and G7 (15-days pre-treatment of Biofield Energy Treated Test formulation), respectively as compared with the disease control group (G2). BDNF level in brain was improved by 5.2% in G7 group as compared with the G4. The level of PGD2 was reported to be significantly (p≤0.001) increased in the G7, G8 (15-days pre-treatment of Biofield Energy Treated Test formulation to the Biofield Energy Treatment per se rats), and G9 (untreated test formulation to the Biofield Energy Treatment per se to the rats) groups by 18.1%, 23.7%, and 30.7% respectively, as compared with the G2. Leptin level was reported to be significantly altered after Biofield Energy Treatment and with the test formulation. Similarly, orexin-A level was also significantly decreased by 37.1% (p≤0.05), 32.6%, 40.5% (p≤0.05), 44.4% (p≤0.05), and 28.2% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2. Ach level was significantly (p≤0.001) decreased by 42.5%, 49.2%, 40.1%, 47.9%, and 45% in the G5, G6, G7, G8, and G9 groups, respectively as compared with the G2. Biofield Energy Healing Treatment (the Trivedi Effect®) per se showed the best results with respect to different efficacy and biomarker parameters in the preventive maintenance group, G6 as compared to the other preventive maintenance groups (G7, G8, and G9) in rat model study. The Trivedi Effect® showed an increased level of melatonin and decreased levels of insomnia related brain biomarkers which might be helpful to induce better sleep in human. It also helped to slow down the disease progression and disease-related complications of the overall animal’s health. These data suggested that Biofield Energy Treatment per se and/or Biofield Energy Treated Test formulation in combination would be the best alternative treatment strategies in order to prevent and protect from the occurrence of any type of diseases. Therefore, the Biofield Energy Treatment might act as a preventive maintenance therapy in order to maintain good health, or full restoration of health or improve the overall health and quality of life in human. This therapy might also reduce the severity of any type of acute/chronic disease (auto-immune related and inflammatory disorders) progression rate and can be used in both before and after the manifestation of any disease symptoms in healthy, unhealthy, and ill peoples such as many thyroid disorders such hyperthyroidism, Goiter, Thyroid nodules, Thyroid cancer, etc. Overall, the data suggested the Biofield Energy Treated test formulation and Biofield Energy Treatment per se showed significant action on thyroid gland with respect to biomarkers, as a Complementary and Alternative Medicine (CAM). This test formulation also can be used against Lupus, Fibromyalgia, Addison Disease, Multiple Sclerosis, Myasthenia Gravis, Aplastic Anemia, Psoriasis, Rheumatoid Arthritis, Crohn’s Disease, Vitiligo, Chronic Fatigue Syndrome and Alopecia Areata, as well as various inflammatory disorders such as Ulcerative Colitis, Dermatitis, Hepatitis, Diverticulitis, Mental Disorders, Parkinson’s and Other Movement Disorders, Stroke and Transient Ischemic Attack (TIA), and in the improvement of overall health and quality of life.
Acknowledgements
The authors are grateful to Dabur Research Foundation, Trivedi Science, Trivedi Global, Inc., and Trivedi Master Wellness for the assistance and support during the work.
References
- 1. (2003) Swedish National Board of Health and Welfare Utmattnings syndrom. Stress relaterad psykisk ohälsa (Exhaustion Syndrome. Stress related mental poor health). (in Swedish) , Stockholm
- 2.Wallensten J, Åsberg M, Nygren Å, Szulkin R, Wallén H et al. (2016) Possible biomarkers of chronic stress induced exhaustion - A longitudinal study. , PLoS One 11, 0153924.
- 3.ter Heegde F, De Rijk RH, Vinkers C H. (2015) The brain mineralocorticoid receptor and stress resilience. , Psychoneuroendocrinology 52, 92-110.
- 4.McEwen B S. (2004) Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. , Ann N Y Acad Sci 1032, 1-7.
- 5.Detka J, Kurek A, Basta-Kaim A, Kubera M, Lason W et al. (2013) Neuroendocrine link between stress, depression and diabetes. , Pharmacol Rep 65, 1591-1600.
- 6.Keller-Wood M(2015) Hypothalamic-pituitary–adrenal axis-feedback control. , Compr Physiol 5, 1161-1182.
- 7.Juster R P, McEwen B S, Lupien S J. (2010) Allostatic load biomarkers of chronic stress and impact on health and cognition. , Neurosci Biobehav Rev 35, 2-16.
- 8.Gulbins A, Grassme H, Hoehn R, Wilker B, Soddemann M et al. (2016) Regulation of neuronal stem cell proliferation in the hippocampus by endothelial ceramide. , Cell Physiol Biochem 39, 790-801.
- 9.Alkadhi K A, Tran T T. (2014) Chronic psychosocial stress impairs early LTP but not late LTP in the dentate gyrus of at-risk rat model of Alzheimer׳s disease. , Brain Res 1588, 150-158.
- 10.Wang J, Yuan J, Pang J, Ma J, Han B et al. (2016) Effects of chronic stress on cognition in male SAMP8 mice. , Cell Physiol Biochem 39, 1078-1086.
- 11.Joshi Y B, Chu J, Pratico D. (2012) Stress hormone leads to memory deficits and altered tau phosphorylation in a model of Alzheimer's disease. , J Alzheimers Dis 31, 167-176.
- 12.Han B, Yu L, Geng Y, Shen L, Wang H et al. (2016) Chronic stress aggravates cognitive impairment and suppresses insulin associated signaling pathway in APP/PS1 mice. , J Alzheimers Dis 53, 1539-1552.
- 13.Galano A, Tan D X, Reiter R J. (2011) Melatonin as a natural ally against oxidative stress: A physicochemical examination. , J Pineal Res 51, 1-16.
- 14.Skene D J, Swaab D F. (2003) Melatonin rhythmicity: Effect of age and Alzheimer's disease. , Exp Gerontol 38, 199-206.
- 15.Lin L, Huang Q, Yang S, Chu J, Wang J et al. (2013) Melatonin in Alzheimer's disease. , Int J Mol Sci 14, 14575-14593.
- 16.Kilduff T S, Peyron C. (2000) The hypocretin/orexin ligand-receptor system: Implications for sleep and sleep disorders. , Trends Neurosci 23, 359-365.
- 17.Sutcliffe J G, L de. (2002) The hypocretins: Setting the arousal threshold. , Nat Rev Neurosci 3, 339-349.
- 18.Peyron C, Tighe D K, AN Van Den Pol, L De, Heller H C et al. (1998) Neurons containing hypocretin (orexin) project to multiple neuronal systems. , J Neurosci 18, 9996-10015.
- 19.Ma Z, Jiang W, Zhang E E. (2016) Orexin signaling regulates both the hippocampal clock and the circadian oscillation of Alzheimer's disease-risk genes. , Sci Rep 6, 36035.
- 20.Nasri H, Baradaran A, Shirzad H, Rafieian-Kopaei M. (2014) New concepts in nutraceuticals as alternative for pharmaceuticals. , Int J Prev Med 5, 1487-1499.
- 21.Rubik B. (2002) The biofield hypothesis: Its biophysical basis and role in medicine. , J Altern Complement Med 8, 703-717.
- 22.Jain S, Hammerschlag R, Mills P, Cohen L, Krieger R et al. (2015) Clinical studies of biofield therapies: Summary, methodological challenges, and recommendations. , Glob Adv Health Med 4, 58-66.
- 23.Evans M, Shaw A, Thompson E A. (2007) Decisions to use complementary and alternative medicine (CAM) by male cancer patients: Information-seeking roles and types of evidence used. , BMC Complement Altern Med 7, 25.
- 24.Gu S, Pei J. (2017) Innovating Chinese Herbal Medicine: From Traditional Health Practice to Scientific Drug Discovery. , Front Pharmacol 8, 381.
- 25.O'Mathúna D. (2001) The best of both approaches. The role of science in complementary and alternative medicine. , EMBO Rep 2(12), 1054-1057.
- 26.Trivedi M K, Branton A, Trivedi D, Nayak G, Mondal S C et al. (2015) Morphological characterization, quality, yield and DNA fingerprinting of biofield energy treated alphonso mango (Mangifera indicaL.). , Journal of Food and Nutrition Sciences 3, 245-250.
- 27.Trivedi M K, Tallapragada R M. (2008) A transcendental to changing metal powder characteristics. , Met Powder Rep 63, 22-28.
- 28.Trivedi M K, Nayak G, Patil S, Tallapragada R M, Latiyal O. (2015) Studies of the atomic and crystalline characteristics of ceramic oxide nano powders after bio field treatment. , Ind Eng Manage 4, 161.
- 29.Trivedi M K, Branton A, Trivedi D, Nayak G, Charan S et al. (2015) Phenotyping and 16S rDNA analysis after biofield treatment onCitrobacter braakii: A urinary pathogen. , J Clin Med Genom 3, 129.
- 30.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) Evaluation of biofield modality on viral load of Hepatitis B and C viruses. , J Antivir Antiretrovir 7, 083-088.
- 31.Trivedi M K, Patil S, Shettigar H, Bairwa K, Jana S. (2015) Phenotypic and biotypic characterization ofKlebsiella oxytoca: An impact of biofield treatment. , J Microb Biochem Technol 7, 203-206.
- 32.Nayak G, Altekar N. (2015) Effect of biofield treatment on plant growth and adaptation. , J Environ Health Sci 1, 1-9.
- 33.Kinney J P, Trivedi M K, Branton A, Trivedi D, Nayak G et al. (2017) Overall skin health potential of the biofield energy healing based herbomineral formulation using various skin parameters. , American Journal of Life Sciences 5, 65-74.
- 34.Singh J, Trivedi M K, Branton A, Trivedi D, Nayak G et al. (2017) Consciousness energy healing treatment based herbomineral formulation: A safe and effective approach for skin health. , American Journal of Pharmacology and Phytotherapy 2, 1-10.
- 35.Branton A, Jana S. (2017) The influence of energy of consciousness healing treatment on low bioavailable resveratrol in maleSprague Dawleyrats. , International Journal of Clinical and Developmental Anatomy 3, 9-15.
- 36.Branton A, Jana S. (2017) The use of novel and unique biofield energy healing treatment for the improvement of poorly bioavailable compound, berberine in maleSprague Dawleyrats. , American Journal of Clinical and Experimental Medicine 5, 138-144.
- 37.Trivedi M K, Branton A, Trivedi D, Nayak G, Plikerd W D et al. (2017) A Systematic study of the biofield energy healing treatment on physicochemical, thermal, structural, and behavioral properties of magnesium gluconate. , International Journal of Bioorganic Chemistry 2, 135-145.
- 38.Anagnos D, Trivedi K, Branton A, Trivedi D, Nayak G et al. (2018) Influence of biofield treated vitamin D3on proliferation, differentiation, and maturation of bone-related parameters in MG-63 cell-line. , International Journal of Biomedical Engineering and Clinical Science 4, 6-14.
- 39.Lee A C, Trivedi K, Branton A, Trivedi D, Nayak G et al. (2018) The potential benefits of biofield energy treated vitamin D3 on bone mineralization in human bone osteosarcoma cells (MG-63). , International Journal of Nutrition and Food Sciences 7, 30-38.
- 40.Stutheit M E, Trivedi K, Branton A, Trivedi D, Nayak G et al. (2018) Biofield energy treated vitamin D3: Therapeutic implication on bone health using osteoblasts cells. , American Journal of Life Sciences 6, 13-21.
- 41.Trivedi M K, Patil S, Shettigar H, Mondal S C, Jana S. (2015) The potential impact of biofield treatment on human brain tumor cells: A time-lapse video microscopy. , J Integr Oncol 4, 141.
- 42.FGD Amaral, Cipolla-Neto J. (2018) A brief review about melatonin, a pineal hormone. , Arch Endocrinol Metab 62, 472-479.
- 43.Gupta Y K, Gupta M, Kohli K. (2003) Neuroprotective role of melatonin in oxidative stress vulnerable brain. , Indian J Physiol Pharmacol 47, 373-386.
- 44.Persengiev S P. (2001) The neuroprotective and antiapoptotic effects of melatonin in cerebellar neurons involve glucocorticoid receptor and p130 signal pathways. , J Steroid Biochem Mol Biol 77, 151-158.
- 45.Hardeland R, Tan D X, Reiter R J. (2009) Kynuramines, metabolites of melatonin and other indoles: The resurrection of an almost forgotten class of biogenic amines. , J Pineal Res 47, 109-126.
- 46.Huang E J, Reichardt L F. (2001) Neurotrophins: roles in neuronal development and function. , Annu Rev Neurosci 24, 677-736.
- 47.Bathina S, Das U N. (2015) Brain-derived neurotrophic factor and its clinical implications. , Arch Med Sci 11(6), 1164-1178.
- 48.Chu C, Wei H, Zhu W, Shen Y, Xu Q. (2017) Decreased prostaglandin D2 levels in major depressive disorder are associated with depression-Like behaviors. , Int J Neuropsychopharmacol 20(9), 731-739.
- 49.Friedman J M. (1998) Leptin, leptin receptors, and the control of body weight.NutrRev56:S38-46.discussion. 54-75.
- 50.Paz-Filho G, Wong M L, Licinio J. (2010) The procognitive effects of leptin in the brain and their clinical implications. , Int J Clin Pract 4(13), 1808-1812.
- 51.Chan J L, Mantzoros C S. (2005) Role of leptin in energy-deprivation states: Normal human physiology and clinical implications for hypothalamic amenorrhoea and anorexia nervosa. , Lancet 366, 74-85.
- 52.Lu X Y, Kim C S, Frazer A, Zhang W. (2006) Leptin: A potential novel antidepressant. , Proc Natl Acad Sci U S A 103, 1593-8.
- 53.Inutsuka A, Yamanaka A. (2013) The physiological role of orexin/hypocretin neurons in the regulation of sleep/wakefulness and neuroendocrine functions. Front Endocrinol (Lausanne). 4, 18.
Cited by (2)
This article has been cited by 2 scholarly works according to:
Citing Articles:
